Single Molecule Biophysics
Dr. Christoph Baumann, Lecturer in Molecular Biophysics
Department of Biology, University of York
Background
Dr. Christoph Baumann, Lecturer at the University of York, Department of Biology and his group work with advanced imaging techniques to push forward our understanding of spatio-temporal dynamics in the bacterial cell envelope. Using a Photometrics camera, the group was the first to observe that, contrary to expectations, proteins in the outer cell membrane don't diffuse significantly when tracked, and that new proteins are inserted predominantly at mid-cell during growth.1 This means that bacteria can very quickly turnover their outer membrane proteins to adapt to new environmental challenges during growth, and this work initiated a new investigation of inter-membrane crosstalk in the Gram negative bacterial cell envelope.
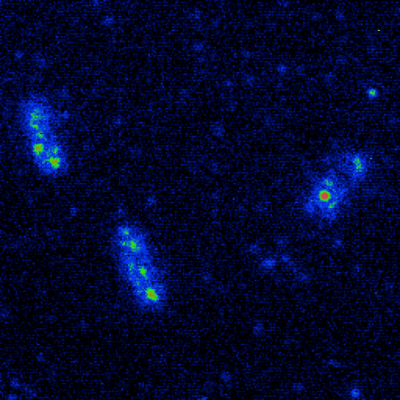
Figure 1: Alexa Fluor 488-labeled colicin E9 protein molecules bound to extracellular face of BtuB
transmembrane receptors in the outer membraneof live Escherichia coli JM83 cells (256 x 256 pixels,
16.9 x 16.9 µm, 30 frame sum, video data collectedwith 33 ms exposure and global shuttering).
Challenge
To pursue this research area, Dr. Baumann and his colleagues use TIRF microscopy alongside laser scanning confocal FRAP microscopy. When tracking single molecules, sensitivity and speed are all-important."Better temporal and spatial resolution means better quality data," Dr. Baumann shares. Previously, the large pixels, slow speed, and the excess noise factor of their EMCCD camera limited both these aspects. Further, the small sensor size and the need to use a 1.6× magnification optic to match pixel size lead to a very small field of view, and using additional lenses lost them precious light.
Better temporal and spatial resolution means better quality data. We’ll definitely be using the Prime 95B Scientific CMOS (sCMOS) camera for our upcoming experiments.
Dr. Christoph Baumann
Solution
The Photometrics Prime 95B Scientific CMOS (sCMOS) camera was the perfect fit for the team's research, with the 11 µm pixels giving optimal resolution paired with the 100× objectives used for TIRF microscopy. The faster speed and higher signal to noise ratio of the back-illuminated CMOS also provided better temporal resolution."This increased resolution is of great benefit for all the research we do, not just this experiment," Dr. Baumann told us."Since the camera is USB3.0, it's very easy to move the camera to other setups for short periods."
The huge field of view of the Prime 95B is not only useful for increasing throughput, but allows the team to use single-camera optical splitting technology. Dr. Baumann concluded,"We're going to use a polarization splitter in an upcoming experiment, and the Prime 95B's chip is large enough to still have a great field of view with both images side by side."
Learn More About The Prime BSI
References
- Supramolecular assemblies underpin turnover of outer membrane proteins in bacteria, P. Rassam et al., 2015, Nature 523: 333-336.
- Intermembrane crosstalk drives inner-membrane protein organization in Escherichia coli, P. Rassam et al., 2018, Nature Commun 9: 1082.