Optogenetic Voltage Clamp Imaging
Prof. Alexander Gottschalk, Ms. Amelie Bergs
BUCHMANN INSTITUTE FOR MOLECULAR LIFE SCIENCES, GOETHE UNIVERSITY, FRANKFURT, GERMANY
Background
Prof. Alexander Gottschalk and his team at Goethe University study the nervous system of the model organism nematode worm Caenorhabditis elegans, specifically mechanisms of transmission at chemical synapses and function of single neurons within the neuronal networks.
We spoke with PhD student Amelie Bergs, “Our research is focused on C. elegans and optogenetics, particularly developing a new method we call optogenetic voltage clamp or OVC, which combines the advantages of imaging with the control capabilities of electrophysiology and voltage clamping.”
“We use a tandem protein consisting of depolarising and hyperpolarising tools fused together, and we combine this with a genetically encoded voltage indicator, specifically QuasAr2. This shows dim fluorescence depending on the membrane voltage of the cell, which we can keep within a desired level by adapting the wavelengths of our light source, so we clamp the fluorescence and the voltage. OVS is a purely optical approach we are using on specific muscle cells of immobilized C. elegans.“
41467_2023_37622_MOESM6_ESM.mp4
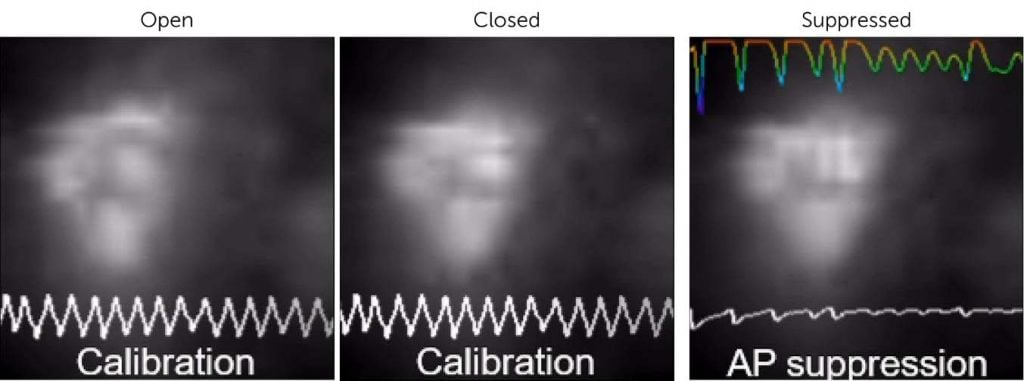
Figure 1: Optogenetic voltage clamp (OVC) of a C. elegans muscle cell, showing the native open and closed morphologies, and then the forced action potential suppression via OVC control. Acquired with the Kinetix22 across an approx. 30×30 pixel ROI. Video in link above image.
Challenge
Voltage signals, even those in the slower firing C. elegans, are rapid and dynamic, requiring the need for high-speed imaging and good sensitivity at low exposure times. Ms Bergs told us more, “We have restrictions in the speed as our GEVI is also rather slow, and we have restrictions from the software side. In the future, we could increase the speed here, but this would be at 1 ms exposure and we would have far fewer photons per frame, so we need high sensitivity to do this.”
“We were previously using an EMCCD, it was difficult to perform clamping due to vibrations from the fan, so we also need a camera that can be water-cooled with no vibrations.”
With custom software scripts for running OVC in Micro-Manager as well, any new device should be compatible with Micro-Manager software for ease of setup and use.
The Kinetix22 is faster and more sensitive, our photon count went up and we can use more speed… I had no issues and I’m very pleased.
Ms Amelie Bergs
Solution
The Kinetix22 is an ideal solution for this application. Featuring high speed. high sensitivity, water cooling, and great software flexibility. The four main operating modes of Sensitivity, Speed, Sub Electron, and Dynamic Range allow for optimizations to be made, whether performing OVC on a small section or imaging an entire nematode worm.
Ms Bergs outlined her experience with the Kinetix22. “With the water cooling there were no vibrations and it makes quite a difference. we got a lot more measurements done. We use the Dynamic Range mode because we have an intense laser signal.”
“It’s even more sensitive and faster. we can even increase the speed in the future. Some issues we had before weren’t a problem anymore. I think the photon count went up too. Of course, in parallel, you’ve got a higher resolution and the camera is future-proof and flexible for other experiments we can do … I’m very pleased with the Kinetix22, I had no issues using it.”
Reference
A.C.F. Bergs, J.F. Liewald. S Rodriguez-Rozada, 0 Liu. C Wirt, A Bessel, N. Zeitzschel. H. Durmaz. A Nozownik. J. Vierock. Cl. Bargmann. P. Hegemann. J.S Wiegert. A. Gottschalk (2023) All-optical closed-loop voltage clamp for precise control of muscles and neurons in live animals. Nat Commun 14, 1939 (2023) https://doi.org/10.1038/s41467-023-37622-6
