diSPIM Light-Sheet
Prof. Matthias Weiss, Ivana Jeremic
Physics of Living Matter, University of Bayreuth, Germany
Background
Prof. Matthias Weiss and PhD student Ivana Jeremic research challenging problems at the interface of physics and biology, focusing on understanding self-organization processes in living organisms. Prof. Weiss told us about his latest project, "We have built a new light sheet microscopy system for imaging dynamic processes within large samples, with Ivana's project focusing on the embryogenesis of transgenic nematode Caenorhabditis elegans until gastrulation. Early C. elegans embryos seem to work on autopilot in terms of self-organization, and we have been successful already in monitoring mechanical cues that drive cell positions until gastrulation. With these data, we were able to even predict cell positions and migration paths via a computational model."
"Now we want to dive a bit deeper and get information on how cells structure themselves internally before undergoing division, so we can learn more about individual steps during embryogenesis that has been missing in our analytical predictions so far." Prof. Weiss and team are using an inverted SPIM (iSPIM) light sheet imaging system in order to observe cell behavior within C. elegans samples throughout development.
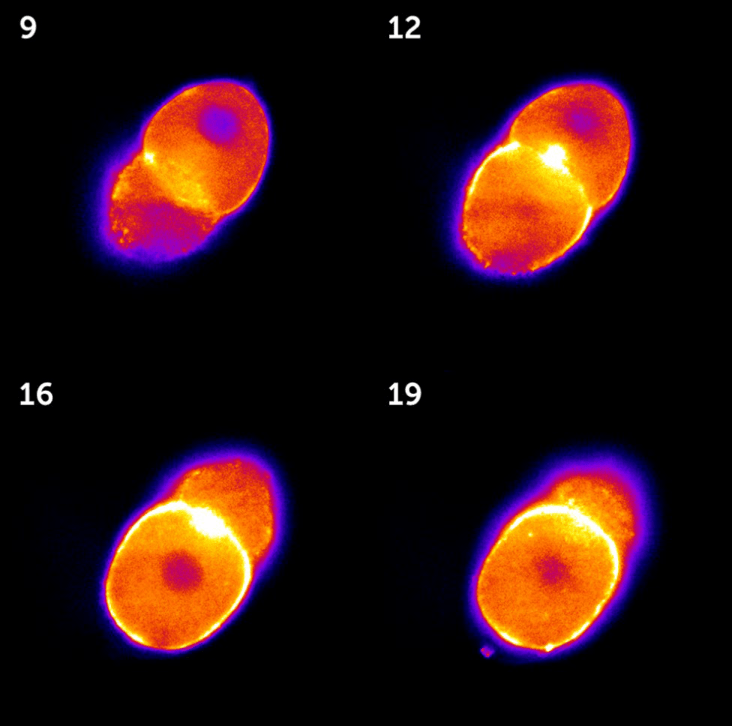
Figure 1: Images taken from a 3D stack of a C. elegans embryo in the two-cell state, acquired with the Kinetix sCMOS.
High-intensity areas represent the actomyosin cortex that exerts chiral forces during cell division.
The numbers on each image represent the z-position within the stack.
Challenge
While light-sheet microscopy is well-suited to imaging large samples with relatively low illumination levels, the light sheet itself requires fine-tuning for the best results. Prof. Weiss told us more about his imaging challenges, "We want a light sheet that is long and thin enough so that we can get the full volume of the worm embryo, which is about 50 μm in diameter. Bleaching is an issue as excess light can poison the embryo. If we used something like laser scanning confocal, the embryo would never go beyond the four-cell stage and would die, so the light sheet with low laser illumination is ideal as we can even observe hatching."
"Some of the fluorescent protein constructs within the sample change their expression during embryogenesis so therefore we have to be able to capture signals from low to high intensity without having to change camera modes, so we need a high dynamic range."
Alongside a sensitive camera with a high dynamic range, this project is also best suited to a detector with a large sensor and a small pixel in order to capture the maximum resolution across the largest field of view.
We were very impressed by the signal to noise ratio and speed we got with the Kinetix!
Prof. Matthias Weiss, Ivana Jeremic
Solution
The Kinetix sCMOS is an ideal solution for this application as well as light-sheet microscopy in general, combining a balanced 6.5 μm pixel with a huge 10-megapixel array across a 29 mm sensor, resulting in high resolution across the whole C. elegans embryo even at lower magnifications.
Prof. Weiss told us about his experience with the Kinetix, "Typically we can take 50 images through the volume of the worm embryo in around 5 seconds, but due to the improved signal-to-noise ratio with the Kinetix we can further tune our experiments and image faster with shorter exposure times so we can also look at more rapid features within the sample."
"Also, with the greater quantum efficiency of the Kinetix, we can tune down the laser intensity so that we can be gentler and keep it more in the native state. In the long term, we intend to also image and rate man-made bio fabricates over days, so we really need the sensitivity in order not to perturb the samples'development."
"We will use dynamic range mode for the good signal-to-noise ratio at the decent speed and ability to image weak and intense signals, we were impressed by the signal-to-noise ratio we got with the Kinetix."

Learn More About The Kinetix
Download This Customer Story