Imaging Mitotic Dynamics
Dr Gary Gorbsky, W.H. and Betty Phelps Chair in Developmental Biology
Program Chair, Cell Cycle & Cancer Biology Research Program
Oklahoma Medical Research Foundation
Background
Research in the Gorbsky lab focuses on the basic mechanisms of how chromosomes assemble and move during cell division in normal cells and in cancer cells, the process termed mitosis. A major emphasis is the mitotic spindle checkpoint pathway that makes sure that each copy of the 23 pairs of chromosomes is distributed equally to each of the daughter cells.
This checkpoint system detects chromosomes that have failed to align at the metaphase plate and delays chromatid separation at anaphase until alignment is complete. Failure of the checkpoint pathway may underlie chromosome abnormalities which are hallmarks of cancer and defects in embryo development.
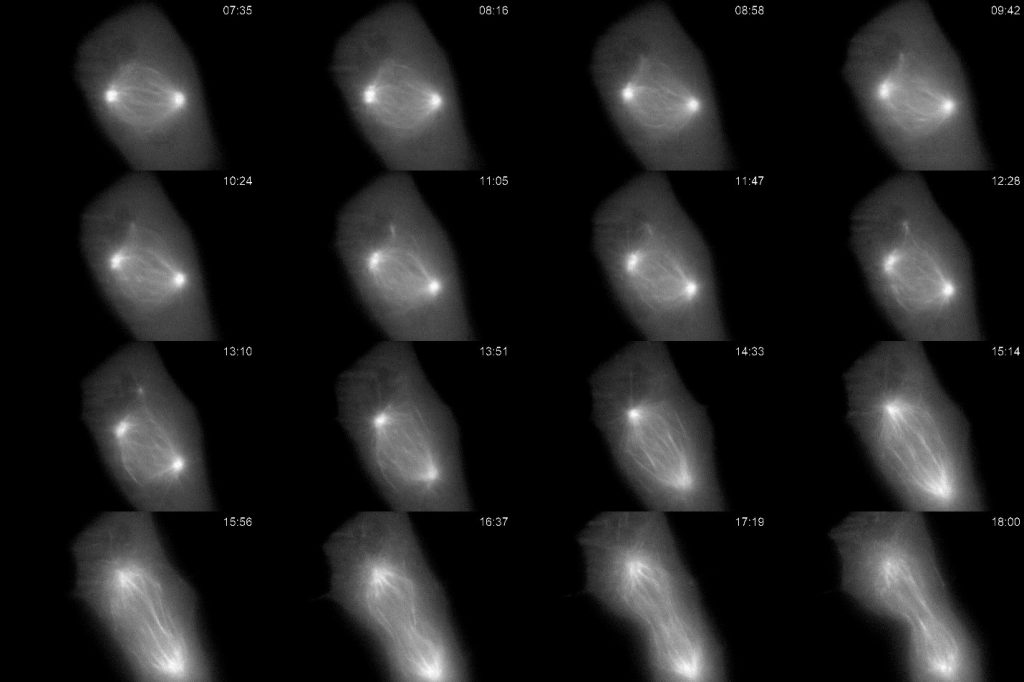
Figure 1: Cultured Xenopus cell expressing GFP-tubulin undergoing mitosis. Imaged every ten seconds for over 18 minutes using light sheet illumination and a high resolution oil immersion objective. Selected frames are shown depicting major changes in the mitotic spindle as the cell progressed from prometaphase through metaphase, and anaphase. The high sensitivity of the Prime BSI allowed for greater total number of samples at lower illumination without noticeable photobleaching or photodamage allowing us to track changes in the mitotic spindle microtubules.
Challenge
The use of fluorescent proteins in live cell microscopy has revolutionized the study of mitosis. However, there are many pitfalls that stem from the tendency of the fluorescent proteins to be photobleached and from cellular damage due to phototoxicity. Mitosis is particularly sensitive to phototoxicity and substantial efforts are taken by the lab to minimize the amount of light reaching the sample.
At the same time, the lab is interested in mapping events at the highest temporal and spatial resolution. Measuring fluorescent protein location during mitosis is challenging, as structures assemble and disassemble quickly. High illumination and emission intensities perturb the mitotic process which requires them to use short exposure times to limit cell damage.
Low excitation light and short integration times result in low photon numbers arriving at the camera, so high camera detection efficiency and low camera noise is key to generating good data.
The use of fluorescent proteins in live cell microscopy has revolutionized the study of mitosis. However, there are many pitfalls that stem from the tendency of the fluorescent proteins to be photobleached and from cellular damage due to phototoxicity. Mitosis is particularly sensitive to phototoxicity and substantial efforts are taken by the lab to minimize the amount of light reaching the sample.
Dr Gary Gorbsky
Solution
The Photometrics Prime BSI, with its small pixels and high quantum efficiency, allows the Gorbsky lab to sample at high spatial resolution with high sensitivity. This allows them to image events in mitosis with finer time resolution or over longer duration which gives them better sampling with less total light input and lower overall photobleaching and photodamage. This is critical with their new light sheet imaging system, where they might acquire through focus series at frequent intervals.
For example, where their previous high resolution microscope and camera could at most image cells at one to five minute intervals for the entire process of mitosis, the Prime BSI coupled to their light sheet microscope enables them to capture images every few seconds, with cells successfully completing mitosis with no ill effects.

Learn More About the Prime BSI