iSIM Live Cell Imaging
Dr. Daniel Dickinson
Department of Molecular Biosciences, University Of Texas, Austin
Background
The Dickinson lab aims to understand the generation of polarity in cells, namely when two ends of a living cell become molecularly distinct from each other. Polarity is vital for the proper function of cells and can become disrupted in diseases such as cancer.
Dr. Dickinson and his team employ a multi-disciplinary approach including fluorescence microscopy of live samples and quantitative image analysis. Working mainly with the embryos of the genetically manipulable model system, the nematode worm Caenorhabditis elegans, the Dickinson Lab uses imaging and single-molecule interrogation techniques, to determine the biochemical interactions that lead to the determination of the direction of the polarity axis and the timing of polarity establishment.
Tracking single molecules expressed at native levels in the C. elegans embryo allows them to measure diffusion constants, and using single-molecule pull-down techniques, establish protein-protein interactions during the biogenesis of cell polarity.
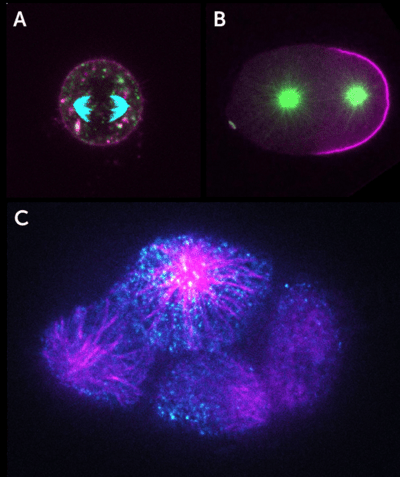
Figure 1: Images of dividing cells taken with the Kinetix22 on a Nikon Ti2 with a Visitech iSIM super-resolution
confocal head. A) A C. elegans zygote, microtubules (green), and cell polarity marker PAR-2 (magneta). B) A dividing
mouse embryonic cell embedded in 3D Matrigel, microtubules (cyan), cell tracking dye (magenta), and apical
polarity marker Podacalyxin (green). C) An 8-cell C. elegans embryo, microtubule TPXL-1 protein (magenta),
and cell polarity determinant PAR-3 (cyan).
Challenge
Tracking single molecules in time and space in living embryos requires a high spatial and temporal resolution from a detector. The embryo is sensitive to total illumination power, and the proteins of interest move in all dimensions of position (X, Y, Z) and time. Capturing the position and temporal information allows the lab to draw conclusions about molecular interactions contributing to the formation of cell polarity.
As Dr. Dickinson pointed out, "Low read noise is really critical". The total amount of light collected is so low in any one frame. Without low read noise, high quantum efficiency, and a uniform camera background, they often couldn't collect enough photons for precise measurements.
The Kinetix22 has a uniform background, high sensitivity and low read noise, allowing us to collect enough photons to make precise measurements.
Dr. Daniel Dickinson
Solution
The Kinetix22 sCMOS provides a unique solution, with both high speed and very low read noise options. Fast particle tracking in Speed mode provides robust data for the calculation of molecular diffusion constants, while Sub-Electron mode allows for precise molecular localization with limited signal thanks to the ultra-low read noise of less than 0.7 e-.
The Kinetix22 combines multiple modes for flexible yet powerful imaging, with Speed, Sensitivity, Sub-Electron, and Dynamic Range modes. Imaging with select modes across a large sensor, more than twice the size of typical sCMOS, allows for high-throughput and efficient imaging.

Learn More About The Kinetix22