What is GFP
Introduction
One of the most important discoveries in the field of fluorescent microscopy was found in a jellyfish in the 1960s. Osamu Shimomura of Princeton University was studying Aequorea victoria, a bioluminescent jellyfish. It should be noted here that luminescence is not the same as fluorescence:
- Luminescence: the spontaneous emission of light from a substance (when that substance is an animal, it is termed bioluminescence)
- Fluorescence: the emission of light from a substance that has absorbed light and becomes excited
Through the study of A. victoria, two major proteins were discovered: aequorin (a photoprotein), and green fluorescent protein (GFP). The jellyfish produces calcium, which interacts with aequorin and produces blue luminescence. This blue light is absorbed by GFP and re-emitted as green fluorescence. These proteins have been isolated and purified from the jellyfish and are used heavily in research to this day. For this research, Osamu Shimomura and colleagues won the Nobel Prize in Chemistry in 2008.
Green Fluorescent Protein
GFP is excited by light in the blue/violet/ultraviolet portion of the spectrum and emits light in the green portion (hence the name). The structure of the protein can be seen in Fig.1. GFP is a barrel shape with the fluorescent portion (the chromophore) made up of just three amino acids. When this chromophore absorbs blue light, it emits green fluorescence.
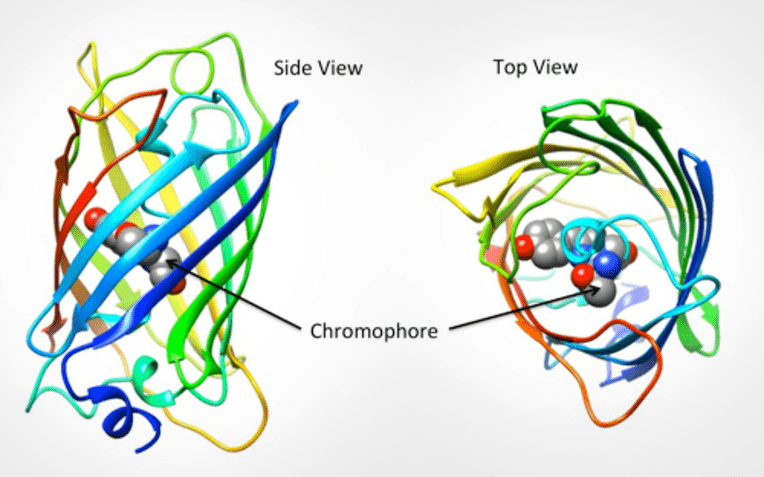
False color has been added to GFP for easy identification of structure. Image from PDB-101: GFP activity page.
GFP In Research
The use for GFP in research became clear once the gene for GFP was also isolated and GFP could be added to cells or genetically grafted into live organisms. Some applications and advantages of GFP discussed below.
GFP as a toxicity marker: due to the fact that GFP decreases in fluorescence intensity with increasing toxicity, it can be used as a marker for environmental toxicity. GFP can be added to host organisms with no negative effect, and then the intensity tracked throughout different environments in various organisms.
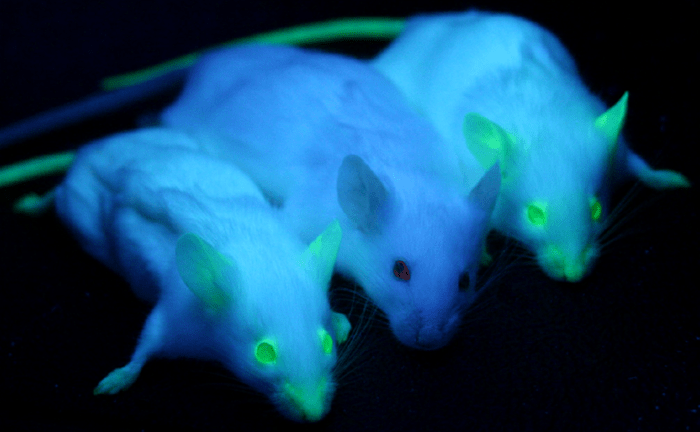
GFP is heritable, if an organism has GFP knocked-in to its genome, GFP will naturally be passed onto offspring without any additional processes, allowing for non-invasive ways of introducing a fluorescent marker and tracking it across generations of animals or cells. GFP does not interfere with any biological processes. Transgenic mice can be labeled with GFP, which is then easily observed in their offspring just by exposing them to blue or UV light, as seen in Fig.2.
GFP can be fused to other proteins, effectively making those proteins fluorescent. This can be done with special linkers so that GFP doesn't affect the function of the protein of interest, and it can still diffuse through cells. This allows any protein to be localized and tracked using standard fluorescent microscopy, by shining a blue light on the cells, the protein of interest will fluoresce back with a green light.
GFP in live-cell experiments: the classic green fluorescent molecule is fluorescein isothiocyanate (FITC), but this is toxic to cells and cannot be used directly without first fixing the cells or causing unavoidable damage. GFP is far less harmful as it is a naturally-occurring protein and can be used in experiments on live cells while causing virtually no damage, especially if it is passed on to offspring.
GFP in advanced microscopy applications. Several fluorescence microscopy applications such as fluorescence recovery after photobleaching (FRAP) and Förster resonance energy transfer (FRET) were developed with GFP, allowing for researchers to use ever more specific and powerful applications of fluorescence for their imaging. These techniques are described in other short articles, namely what is FRET and what is FRAP?
GFP is modifiable, as the genetic and amino acid code for GFP is well understood it has been subject to several modifications. Firstly GFP was modified to produce enhanced GFP (eGFP), which has increased fluorescence intensity, greater photostability, more convenient excitation peaks and higher efficiency at room temperature. Modifications directly to the chromophore allow for GFP to fluoresce with different colors, creating blue (BFP), cyan (CFP), yellow (YFP), red (RFP) and others, all of which have been improved upon separately and have their own applications. Some standout modifications include mCherry (red), Citrine and Venus (yellow), and Cerulean (cyan) to name a few. Entire families of fluorescent proteins now exist, all derived from the original GFP, as seen in Fig.3.
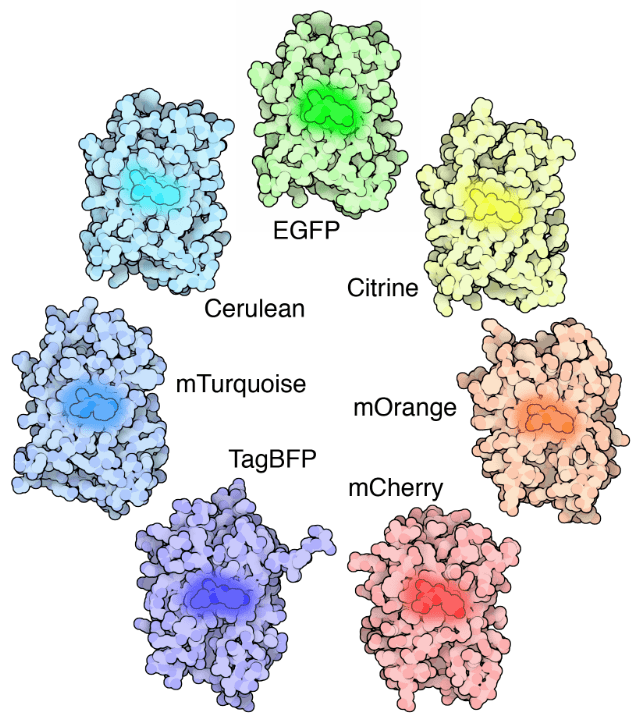
Summary
GFP is a fundamental part of fluorescence microscopy due to the ease of use and the applications being limited only by the researcher's imagination. Constant improvements on GFP over time have caused fluorescence microscopy and research to move forward, due to the highly flexible nature of GFP and the large body of research based on using GFP and its many variants.
Further Reading
Back To Microscopy Basics
Join Knowledge and Learning Hub