Confocal Raman Microscopy Study of Biological Tissues
Introduction
Over the past decade, significant progress has been achieved in the development of high-precision spectrographs, scientific camera technologies, and novel data analysis algorithms. These advances make Raman spectroscopy a practical and powerful tool for complex biological cell- and tissue-related research. The non-invasive, simple, fast, and highly reproducible characteristics of Raman spectroscopic measurement make it a strong candidate for several high-profile applications like cancer diagnostics, microbiology identification, and anti-counterfeit food and drug screening.
Although compact and benchtop Raman spectrometers are relatively affordable for many research and industrial laboratories, integrated confocal Raman microscopy systems, which are arguably one of the most desired tools for tissue analysis, still command high prices that make them more difficult to acquire for small and medium-size institutions and laboratories with limited funding.
In this article, we demonstrate that a standard optical microscope can be converted to a high-performance confocal Raman microscope using a compact Teledyne Princeton Instruments IsoPlane® 81 imaging spectrograph.
Experimental Setup
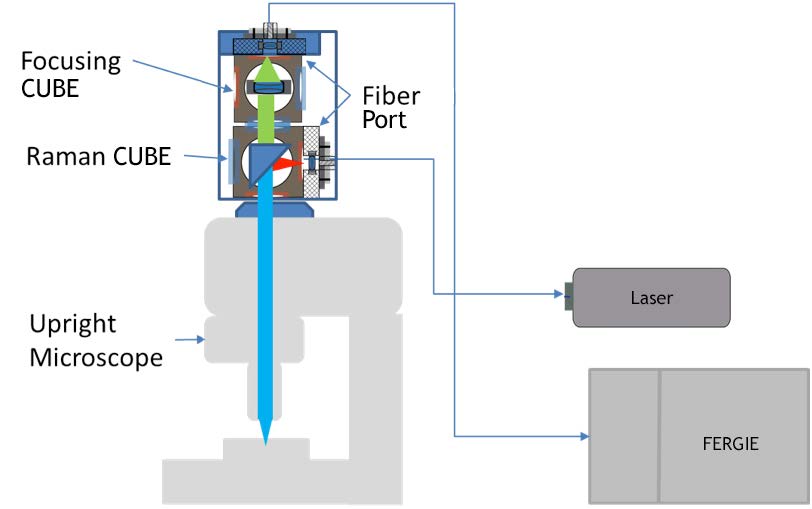
The schematic of the confocal setup is shown in Figure 1. The coupling optical assembly is built with two IsoPlane 81 pre-aligned CUBES (Raman and Focusing). Two fiber ports with FC adapters are used for laser and signal collection fibers. A single-mode 532 nm laser is used as the excitation source. Raman scattering is fiber coupled to an IsoPlane 81 imaging spectrograph. The coupling assembly is connected to an Olympus BX51 upright microscope using a standard camera port adapter. The proprietary CUBE-based coupling is easy and requires minimal alignment effort. Confocality is achieved via careful selection of the core size of the coupling optical fibers.
Samples of brain tissue and mitosis-Ascaris egg were used for the measurements. The samples were deposited on standard microscope glass slides.
Results
Figure 2 shows the 532 nm Raman spectra collected at different relative positions between the sample plane and the microscope objective with an exposure time of 2 sec. A step size of 2 µm was used to measure the depth profile.
Figure 2A shows the representative spectra at 10 µm steps. Figure 2B is the background-corrected intensity profile of the peak at ~2950 cm-1. Figure 2C is the color microscope image of the sample. The broad background arises from the fluorescence of the glass substrate. Features at ~1400, ~1670, and ~2950 cm-1 from the sample are clearly visible.
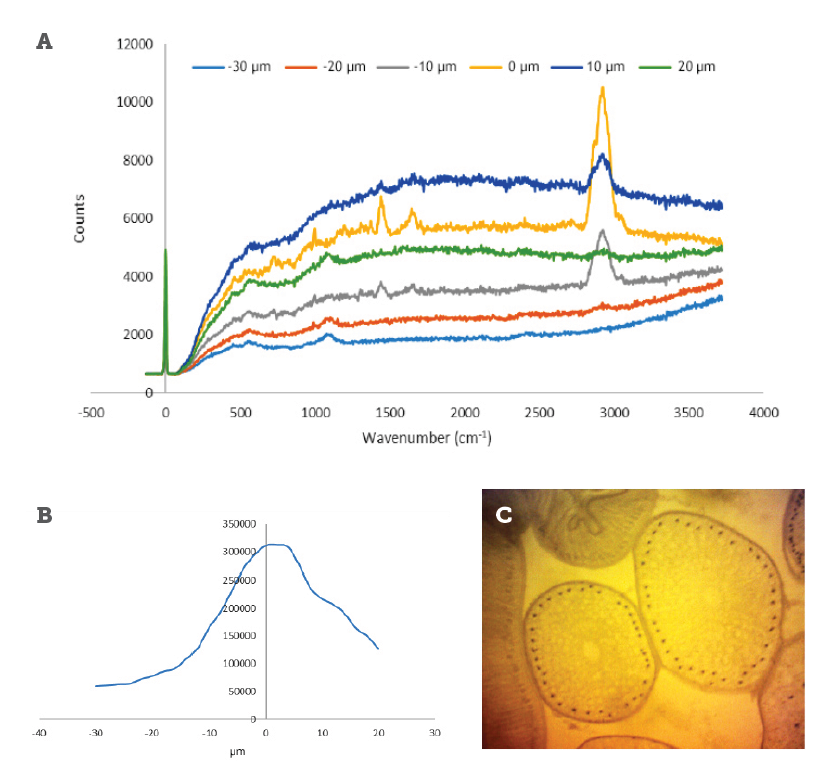
Based on the optics used in this setup, we estimated the lateral spatial resolution to be 1.3 µm and the FWHM depth resolution to be 4.2 µm. In practice, many factors will have an impact on these numbers, including the type and thickness of the cover glass, the transparency of the sample, and the thickness, refractive index, and optical properties of the immersion oil. (A dry objective was used in the current study.)
Figure 3 shows the 532 nm Raman spectra of dried and buffered brain tissue, respectively. The spectrum of brain tissue in buffer is dominated by water Raman signatures at ~3450 and ~1700 cm-1. Raman peaks in the CH/NH stretch and fingerprint region from both samples can be clearly observed.
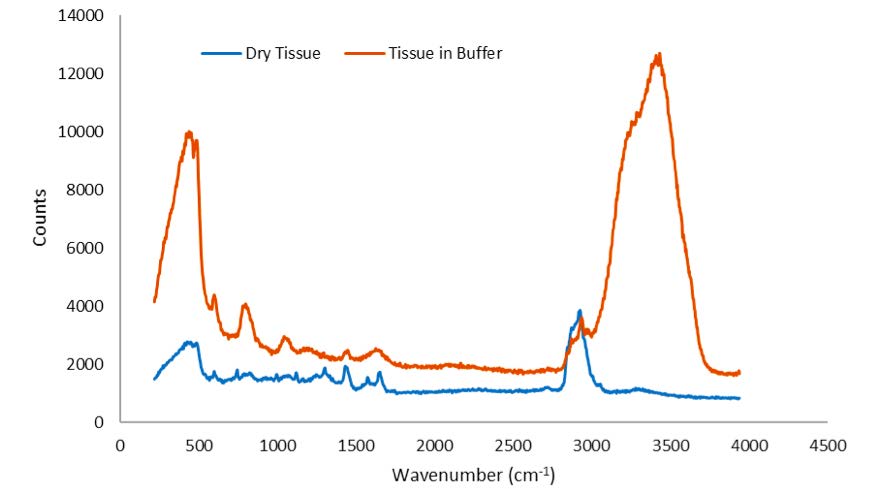
Figure 3. The 532 nm Raman spectra of air-dried brain tissueand brain tissue in aqueous buffer.
Conclusion
We have demonstrated a confocal Raman microscopy setup using a standard upright microscope. Excellent spatial and depth resolution were achieved. Combined with the IsoPlane 81 scientific imaging spectrograph, high-quality Raman spectra were collected in a relatively short exposure time. The design of the coupling optics can easily be used on other upright or inverted microscopes. For a microscope with a built-in tube lens, a matching tube lens will be required (as shown in Figure 4).
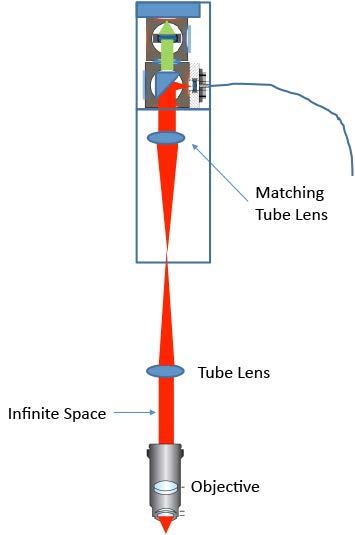
The lateral and axial resolution of the current setup strongly depends on the optics used. Higher resolution can be achieved by carefully choosing the proper fiber collimator, fiber core size, focusing lens, and objective.
We expect this simple and easy-to-use confocal design to benefit many researchers in the field.
Acknowledgment
Brain tissue samples courtesy of Dr. Koseki Kobayashi-Kirschvink, Broad Institute (Cambridge, MA).