Live Cell Dynamics
Prof. Cornelia Monzel
Faculty of Mathematics and Natural Sciences, Heinrich Heine University Düsseldorf, Germany.
Background
The group of Prof. Cornelia Monzel aims to understand the interplay of physical and biological mechanisms that give rise to relevant cellular functions for use in diagnostics and therapy. To do this they make use of label-free and fluorescent methods to investigate membrane fluctuation dynamics and cell signalling pathways.
One technique used by the group of Prof. Monzel is label-free reflection interference contrast microscopy (RICM) in order to look at the dynamics of membrane interactions [1-3]. This technique works by observing the light reflection at micro-structured interfaces, acquiring fast recordings (10 ms) of membrane fluctuations, and reconstructing the interference intensity pattern of bio-membranes into 2D height profiles, as seen in Figure 1. The membrane topography and its dynamics provide information about the strength and type of interaction governing adhesion.
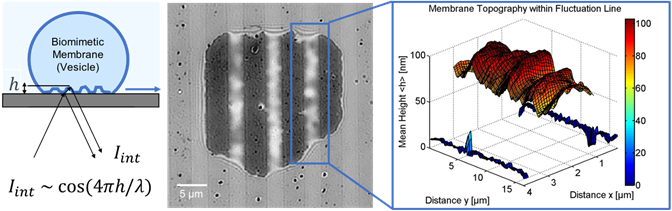
Figure 1: Biomembrane adhesion to microstructured interface. Left) sketch of biomimetic membrane (vesicle) adhering
to a microstructured substrate, mimicking adhesion to a limited number of binding sites. Middle) The contact area is
visualized with the interferometric technique RICM. Right) The detected interference intensity I_int is converted into
membrane topography with nanometric resolution. Images from [1-3].
Another interest of Prof. Monzel's group is imaging the dynamics of cell signalling pathways involved in apoptosis (programmed cell death). To do this, they perform time-lapse imaging of live cells that are undergoing apoptosis, once the receptor CD95 in the plasma membrane gets activated by the CD95-Ligand. The group demonstrated that the process of apoptosis involves clustering of the CD95 receptors, as seen in Figure 2. The group is trying to decipher how the signal activation depends on receptor number and accumulation state, as well as on the dynamics of other molecules in the signalling pathway ultimately leading to apoptosis.
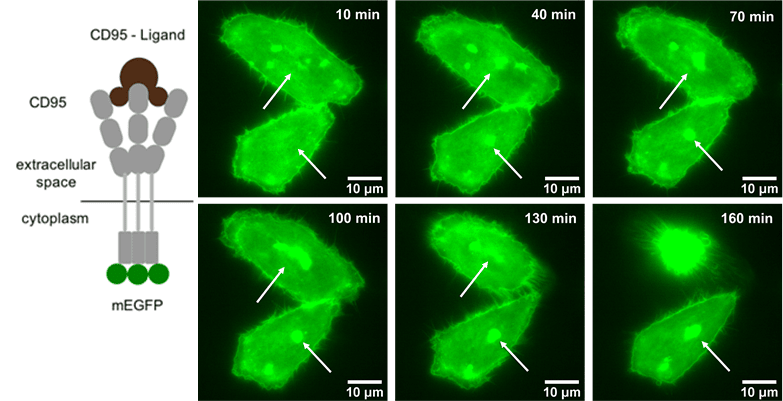
Figure 2: CD95 activation and accumulation triggers cell apoptosis. Left) sketch of CD95 receptors activated by CD95-Ligand. A mEGFP fluorophore is genetically fused to the cytoplasmic domain of the receptor. Right) Temporal dynamics of CD95 accumulation followed by
cell apoptosis monitored in the fluorescent EGFP channel, taken with the Prime BSI.
Challenge
Interference microscopy and RICM provide a challenge in that the techniques requires a high contrast and signal to noise ratio. At the same time, the imaging needs to be fast in order to record membrane dynamics with 10 ms resolution.
When performing time-lapse imaging a large number of cells need to be recorded to ensure a representative sample of the population is captured. High and precise fluorescence signal acquisition is further required to enable ratiometric fluorescence analysis of molecular accumulation.
“Due to the back illumination and 95% quantum efficiency [of the Prime BSI], the sensitivity and efficiency were massively increased.”
Cornelia Monzel
Solution
The group of Prof. Monzel is now using the Teledyne Photometrics Prime BSI for acquiring both fast recordings of biomimetic membrane dynamics and time-lapse images of receptor clustering.
Prof Monzel told us, "there have been massive improvements in sCMOS technology over the past 5 years, especially due to the back illumination and 95% Quantum Efficiency, the sensitivity and efficiency was massively increased". Prof Monzel went on to say that, "with the BSI we can now record a lot of cluster formation. I appreciate the large field of view with 4.2 Megapixel as it is good for getting high statistics during a short amount of time. The small 6.5 µm pixel size here enables resolving clusters at lower magnifications".

Learn More About The Prime BSI
References
- Monzel C., Schmidt D., Kleusch C. et al. (2015) Measuring fast stochastic displacements of bio-membranes with dynamic optical displacement spectroscopy. Nat Commun 6, 8162. https://doi.org/10.1038/ncomms9162
- Monzel C., Schmidt D., Seifert U., Smith A.S., Merkel R. and Sengupta K. (2016) Nanometric thermal fluctuations of weakly confined biomembranes measured with microsecond time-resolution. Soft Matter, 12, 4755-4768
- Zhang, Y., De Mets, R., Monzel, C. et al. (2020) Biomimetic niches reveal the minimal cues to trigger apical lumen formation in single hepatocytes. Nat. Mater. 19, 1026–1035. https://doi.org/10.1038/s41563-020-0662-3
- Gülce S. Gülcüler Balta, Cornelia Monzel, Susanne Kleber, et al. (2020) 3D Cellular Architecture Modulates Tyrosine Kinase Activity, Thereby Switching CD95-Mediated Apoptosis to Survival. Cell Reports 29, 8. 2295-2306. https://doi.org/10.1016/j.celrep.2019.10.054.