High Content Imaging at the Radcliffe Department of Medicine
Dr. Christopher Toepfer
Radcliffe Department of Medicine, University of Oxford, UK
Background
Dr. Chris Toepfer is a principal investigator interested in understanding how cardiac physiology changes in inherited cardiovascular conditions. The lab uses fluorescence microscopy and calcium imaging to observe how cellular contractility is affected across different heart conditions, such as those seen in professional sports.
Using human stem-cell-derived cardiomyocytes with a known genome, they then use CRISPR Cas9 to insert patient-specific mutations and GFP tags into this cell model and observe the effects; such as whether the cells beat harder or faster than normal. This is done with calcium imaging to monitor calcium flux of a cell, and imaging of the GFP-labelled sarcomeres themselves, enabling Dr. Toepfer and team to measure contractility on the fundamental unit of muscle contraction in real-time.
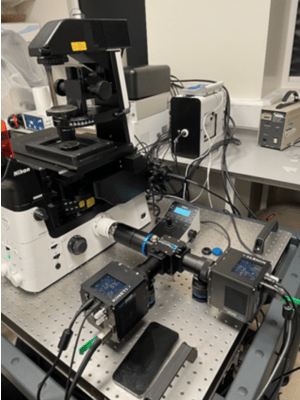
high-content imaging.
Challenge
The sarcomere is a challenging sample to image, as described by Dr. Toepfer: "There are often hundreds of sarcomeres and we need to track each individual one in every single cell, and we do this across hundreds of cells so this is high throughput... Unfortunately, the GFP-tagged protein is only seen a couple of times in each sarcomere, and a sarcomere is only 2.1 µm in size, so we are limited by the availability of light."
"The cells beat once or twice a second and the contraction is very fast, so we need a high framerate to see and track the movement... the initial contraction happens over 50-100 ms and we want to capture as many frames in that short period of time as we can, to really characterize how the contraction and relaxation occur."
The requirement for capturing many rapid events over a large imaging area makes this a very demanding experiment; one that is often limited by the hardware being used. The previous microscope had a 22mm FOV, and the EMCCD cameras on the system were limited to an even smaller imaging area.
In addition, imaging calcium with RFP and sarcomeres with GFP requires the use of multiple channels, meaning each acquisition takes longer. Dr. Toepfer mention his previous system for imaging these samples, saying "With our previous system we could only do around 30 fps in a single channel", which was due to the speed limitations of EMCCD technology.
The Kinetix is great, the field of view being larger is very helpful for us, our acquisitions are a lot quicker so we can have a lot more experiments done in the same amount of time, and get a lot more data, which is also of a higher quality
Dr. Christopher Toepfer
Solution
The new system provides the lab with the capabilities to overcome all of the previous limitations: a larger imaging area, higher acquisition speeds and overall a higher throughput of data. The combination of the Kinetix and the Nikon Ti2 means that the entire 25 mm can be imaged at ~600 fps which is 20x more data than the previous system.
Dr. Toepfer told us about his experience with the Kinetix, "The Kinetix is great, the field of view being larger is very helpful for us so we can capture multiple cells now, our acquisitions are a lot quicker so we can have a lot more experiments done in the same amount of time and get a lot more data, which is also of a higher quality."
"The Kinetix is allowing us to look at rapid events... now we are running at 100 fps compared to the previous 30 fps, and if we shrink the chip we can go to over 1000 fps, which now rivals other techniques used to image calcium such as photomultiplier tubes, but on a microscope." Dr. Toepfer also wishes to measure and correlate the action potentials that trigger calcium release upon contraction, using fluorophores for voltage imaging. These voltage events occur far faster than the contraction cycle, and would require even higher speeds to image, easy obtainable with the Kinetix.
The lab is currently using two Kinetix cameras in combination with the TwinCam emission splitter from Cairn Research. In addition to the increased throughout provided by a new microscope and new camera, the TwinCam means that they can now image across multiple fluorescent channels in real-time, allowing for the capture of both calcium waves and sarcomere contractions and correlation
between these two channels.

Learn More About The Kinetix
Download This Customer Story