iSPIM and diSPIM
Introduction
Light sheet microscopy overcomes the challenges of imaging physiological processes. Light exposure can result in phototoxic effects and photodamage on biological samples, which can disrupt cellular functions. Light sheet microscopy uses two perpendicular objectives, one for illumination and the other for detection, and a z-stack of images is collected by sweeping the light sheet through the sample.
Widefield microscopy illuminates samples throughout the axial direction, which excites out-of-focus fluorescence and decreases the contrast of the in-focus signal. Minimizing out-of-focus light reduces the background signal and images with high contrast are comparable to confocal microscopy. Less light exposure decreases the photodamage on living samples, which allows the imaging of large and sensitive samples in 3D over a long period of time.
Single-view inverted selective plane illumination microscopy (iSPIM) is a light sheet microscopy technique that utilizes planar light sheets and widefield detection. It was created as a collaboration between Hari Shroff at NIH and Applied Scientific Instrumentation (ASI). A variant of iSPIM is dual-view iSPIM or diSPIM. Full instructions on how to build a diSPIM in any standard laboratory can be found in Abhishek et al. (2014).
iSPIM
In iSPIM, a laser beam is focused in one direction by a cylindrical lens and objective to form a thin planar light sheet a few micrometers thick. The planar light sheet has a hyperbolic profile with a beam waist that has the most tightly focused mid-point of the illumination profile. The sample should fit within the beam waist to ensure even illumination because light expands away from the waist.
The iSPIM "head" can be mounted on an inverted microscope with two objectives that dip into a reservoir filled with media to image samples. Live samples can be imaged over long periods of time with easy access for media changes. Compared to most other SPIM techniques that require samples to be embedded in a gel and suspended between objectives, preparation for iSPIM is simple and the sample remains stationary. Adherent cells can be cultured on coverslips and whole organisms or tissues can be mounted to coverslips. In addition, transparent tissues can be imaged in the aggressive solvents used in clearing solutions with special cleared tissue objectives.
diSPIM
iSPIM is similar to most microscopy systems where the axial resolution is 2-3 fold worse than the lateral resolution. The dual-view inverted selective plane illumination microscopy (diSPIM) system creates 3D images by collecting volumes from perpendicular directions and computationally combining data from the lateral views to create a volume with isotropic resolution (330 nm) in x, y, and z. A diSPIM system is shown in Fig.1.
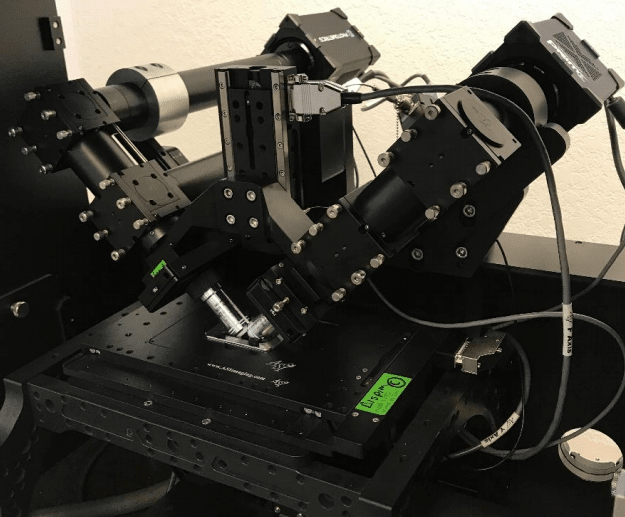
Figure 1: diSPIM on an inverted microscope stand. The sample is mounted onto a coverslip and placed
into a specialized reservoir that is be filled with media. Two perpendicular objectives are dipped into the
reservoir. Each light path as a laser scanner and an sCMOS detector that is moved during a z-stack
by a piezo stage.
Poor axial resolution from one view is overcome by information from the other view. The "head" contains z piezos to ensure the detection objective moves as the light sheet sweeps in the plane of focus. The initial alignment of the diSPIM may seem challenging, but realignment takes approximately 10 minutes. The diSPIM is ideal for 3D imaging of live samples because volumes are quickly acquired and consistently in focus. The system can be bought as a standalone microscope which means that it isn't necessary to have an existing microscope to use it. Neither does the system require any special camera modes or extra features, making the system very simple to implement.
diSPIM Camera Choice
The diSPIM system provides high spatiotemporal resolution imaging in all three dimensions. The observed plane is illuminated, and images can be acquired at 200 fps which means that a camera capable of fast speeds is preferred.
Unlike conventional light sheet microscopy which uses low magnification objectives, diSPIM is typically performed with 40x magnification objectives so a camera with a mid-range (~6.5 µm) or small (~4.25 µm) pixel size would have the desired sampling for high-resolution images.
The signal to noise ratio using diSPIM is comparable to spinning disk confocal so using a camera with high sensitivity would deliver very high-quality images. Light sheet microscopy often uses red and infra-red light to image deep into cells and tissues so it's important that the camera has high quantum efficiency at these wavelengths.
Finally, having a camera with a large field of view would allow the user to more easily image large samples without needing to stitch multiple images together. Alternatively, for smaller samples, the large field of view can be split to perform multi-channel imaging easily on the same sensor.
Download As PDF
References
Abhishek, K., Wu, Y., Christensen, R., Chandris, P., Gandler, W., McCreedy, E., Bokinsky, A., Colon-Ramos, D. A., Bao, Z., McAuliffe, M., Rondeau, G. & Shroff, H. (2014) Dual-view plane illumination microscopy for rapid and spatially isotropic imaging. Nat Protoc. Nov; 9(11): 2555-2573; doi: 10.1038/nprot.2014.172