oSPIM and doSPIM
Introduction
Conventional fluorescence microscopy uses high-intensity light to illuminate the sample, but this excites all fluorophores in the light path, not just the plane of interest. The result is that light emitted from outside the focal plane contributes to the image. Confocal microscopy overcomes this problem by using pinholes to selectively collect light only from the plane of interest. However, high-intensity light still penetrates through the entire sample which causes photobleaching and photodamage.
Light Sheet Fluorescence Microscopy (LSFM) or Selective Plane Illumination Microscopy (SPIM) only illuminates the plane of interest which allows us to collect information from a single plane while also minimizing photobleaching and photodamage to the rest of the sample. By eliminating out-of-focus light in this way, lower light intensities can be used to excite the sample which further contributes to the reduction in photobleaching and photodamage, allowing us to image for extended periods of time. More exposures can, therefore, be taken using LSFM than any other form of fluorescence microscopy.
Limitations Of Conventional Light Sheet Microscopy
Conventional LSFM is performed with two objectives oriented orthogonally (at 90°) to each other so that one objective introduces the light sheet and the other detects the fluorescence signal. However, this orthogonal orientation requires the detection objective to be placed slightly away from the sample to prevent the two objectives from colliding in space. Therefore, a long working distance detection objective is necessary which means that high NA, oil-immersion objectives are incompatible with the conventional LSFM design. This presents a problem for the detection of cellular or subcellular structures that require a high NA detection objective for the superior resolution and light collection efficiency.
Oblique SPIM (oSPIM) overcomes this problem by changing the objective orthogonal orientation and instead, generates the light sheet at an oblique angle using an oil-immersion objective below the sample. A high NA, water dipping, detection objective sits above the sample and is tilted 60° perpendicular to the light sheet which also provides an ideal geometry for cell and tissue culture samples. oSPIM is a platform for high-resolution light sheet microscopy, combining the low photobleaching and photodamage of LSFM with high magnification, high NA objectives for cellular and subcellular imaging. A variant of oSPIM involves two objectives, namely dual-view oSPIM (doSPIM).
oSPIM
The light sheet is formed by the illumination objective below the sample which generates the light sheet at an oblique angle, 30° from the lens. This is achieved simply by steering the input beam off-center in the objective back aperture, partway to total internal reflection (Fig.1).
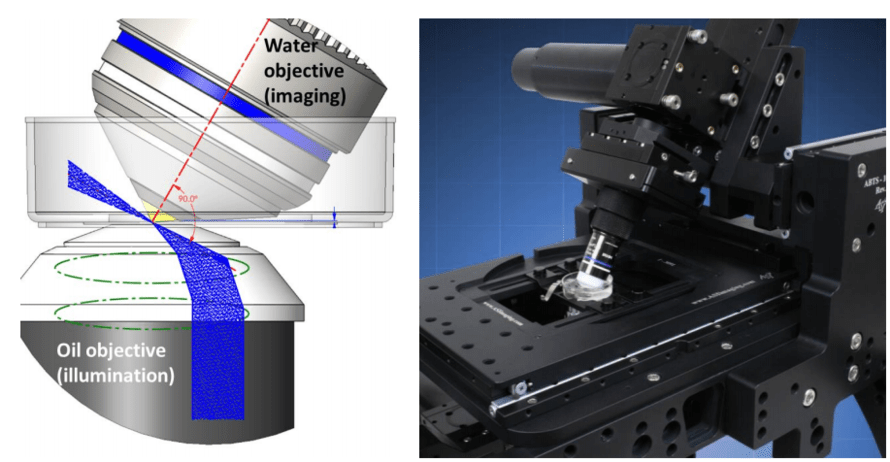
Figure 1: On oSPIM. The left image shows oSPIM objective geometry, while the right image shows oSPIM implemented on aASI RAMM frame.
The high NA oil-immersion objective below the sample can also be used for conventional fluorescence imaging such as widefield, confocal or TIRF. In this way, the oSPIM system is two microscopes in one, combining high-resolution fluorescence microscopy with high-resolution light sheet fluorescence microscopy in one system.
doSPIM
The oSPIM does not give isotropic resolution as the light sheet is only introduced from one side. However, a modified version of the oSPIM has been created which uses a second tilted water-dipping objective below the sample, providing dual-view oblique SPIM. Like in oSPIM, the light sheet is generated at an oblique angle, 30° from the lens. However, the light sheet can now be generated through both objectives sequentially like diSPIM. By imaging the sample from two sides, excellent resolution can be achieved in 3D (below 300 nm in each direction but not quite isotropic because of the non-orthogonal views). The big advantage of doSPIM is that it is capable of providing nearly isotropic resolution using high NA objectives for cellular and subcellular imaging which isn't possible with conventional LSFM. However, the ability to use the system for conventional fluorescence microscopy is lost because the bottom objective is tilted. Furthermore, doSPIM requires mounting samples on thin transparent membranes.
oSPIM and doSPIM Advantages
The primary advantage of oSPIM and doSPIM is applying the benefits of SPIM to imaging cellular and subcellular structures. Conventional and confocal microscopy are invaluable tools for cell biology, but light sheet microscopy allows far longer acquisitions due to greatly reduced photobleaching and photodamage. Higher NA objectives also collect more emission light than conventional LSFM objectives which means that an even lower illumination intensity can be used. This allows cell biologists to follow processes over much longer time scales than previously possible.
The system is typically sold as a standalone microscope which means that it isn't necessary to have an existing microscope to use it. Neither does the system require any special camera modes or extra features, making the system very simple to implement. The oSPIM has the added advantage of being able to perform conventional widefield, confocal and TIRF microscopy with standard 35 mm glass-bottom dishes, increasing the versatility of the system. However, if optimizing resolution is more important, the doSPIM is an attractive alternative.
oSPIM and doSPIM Camera Choice
Unlike conventional light sheet microscopy, oSPIM and doSPIM are performed with high magnification objectives so larger pixel cameras can be used. A larger pixel improves sensitivity which will positively impact cell lifetime by reducing the harmful effects of photodamage and photobleaching. The goal of light sheet microscopy is to image dynamic events in live cells over long time scales so combining a large pixel with a high quantum efficiency will allow for light intensity to be reduced even further.
A larger field of view camera is also beneficial as it allows large sample areas to be imaged without needing to stitch multiple images together. Alternatively, the large field of view can be used with an image splitter to perform multichannel imaging easily on the same sensor.
Download As PDF
Further Reading
Back To Light Sheet
Research Applications
Join Knowledge and Learning Hub