Optical Tweezer Light-Sheet Microscopy
Prof Shiaulou Yuan, Dr. Mohammed Mahamdeh
CARDIOVASCULAR RESEARCH CENTER, MASSACHUSETTS GENERAL HOSPITAL, HARVARD MEDICAL SCHOOL, BOSTON, USA
Background
Professor Shiaulou Yuan runs a lab at the Massachusetts General Hospital, primarily focused on studying congenital heart disease using zebrafish models, looking in particular at the development of cilia, small hairlike structures found in the developing heart.
Prof. Yuan told us more, “Cilia are these little hair-like structures on the surface of cells, we are investigating the function of cilia signaling in zebrafish cardiac development using microscopy techniques, we use a combination of in vivo optical tweezers and custom light sheet microscopy.”
“We image the live heart with light-sheet while we mechanically manipulate cilia with optical tweezers, measuring the response. We target a genetically encoded calcium indicator (GECI) such as GCaMP into these cilia to track their signaling as we manipulate them.”
Prof. Yuan and the team recently published their findings in Science, describing how cilia function as calcium-mediated mechanosensors that instruct left-right asymmetry in the zebrafish embryo (Djenoune et al. 2023).
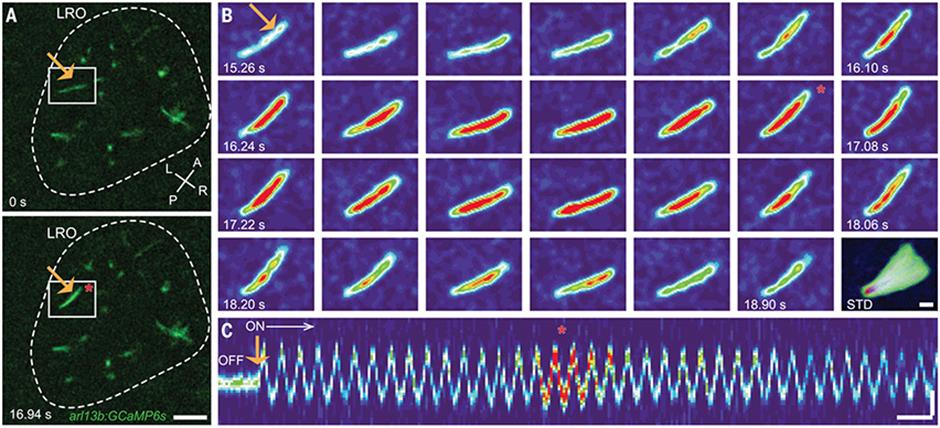
Figure 1:Oscillatory mechanical stimuli on left-right orientation (LRO) cilia activate intraciliary calcium transients. (A) Representative images of the LRO of a c21orf59 morphant zebrafish (scale: 10 μm). (Bl Representative montage of the GCaMP6s- positive LRO cilium highlighted in (A). Scale: 2 μm. Images taken with Prime 958 CMOS cameras. Video example in link above image.
Challenge
Dr. Mohammed Mahamdeh is a biophysicist in the lab of Prof. Yuan, and he described some of their imaging challenges, “In our system, we resolve the whole zebrafish embryo, this sample is around one millimeter in diameter, but the cilia structures are 50 micrometers in diameter. We want to see the cilia protrusions so we can target them with GCaMP and the optical tweezers.”
“In our custom light sheet system, we use a 60x immersion objective. Our aim is to do high-magnification imaging with high numerical aperture lenses. We also want to image at high frame rates to capture the calcium signals and to see how the shape of the cilia changes when they are active. At this point, we need a camera that is sensitive and fast, with the right pixel size.”
Prof. Yuan outlined more challenges, “These cilia are very small, and the signal from each individual cilia is really low, the light regime we are using is very photon starved.”
Overall, this application requires a highly sensitive camera that can image at high frame rates across a large field of view with sub-cellular resolution, all in order to capture functional activity and motion from the small-scale cilia.
The Photometrics cameras are really essential to this research… the [Prime] 95B works really well for us, the pixel size and 95% efficiency are really key in detecting changes in our images.
Prof. Shiaulou Yuan
Solution
Back-illuminated sCMOS cameras are the new modern paradigm of imaging research, and for his work, Prof. Yuan is using multiple Prime 95Bs and Prime BSls for their reliability and sensitivity. A pair of Prime 95Bs is used with the zebrafish models for simultaneous multichannel imaging.
Prof. Yuan explained his choices, ‘The Photometrics cameras that we are using are really essential to this research. We tested several different cameras and demoed everything out there. and we have some Prime BSls and Prime 95Bs. We’d also like to check out the Kinetix!”
“The 95B works really well for us, it has the right pixel size. 95% efficiency was really key to detecting changes in the image when we apply the optical tweezers in vivo, and with the 60x objective we could really resolve the individual cilia.”
Dr. Mahamdeh added, ‘The cameras were easy to set up on our custom system with the C mount and support stands, it was very straightforward!”
