Calcium Imaging at Heidelberg University
Marc Freichel, PhD, Prof.
Volodymyr Tsvilovskyy, PhD.
Heidelberg University
Background
Dr. Freichel's group are primarily interested in how ion channels regulate the influx of calcium ions (Ca2+) as messengers for cellular and systemic functions.
The group is mainly interested in understanding transient receptor potential (TRP) and Ca2+channels and their role in calcium signaling in cells of the cardiovascular system (endothelial cells, smooth muscle cells, cardiac fibroblasts, platelets), epididymal cells and mast cells. This gives the group important information about the corresponding integrative body functions such as vascular and cardiac contractility, transmitter secretion, blood pressure regulation, blood vessel formation, fertility and mast cell activation as well as their role during maladaptive cardiac remodelling and for the development of diabetes mellitus associated long term sequelae.
The laboratory uses transgenic approaches such as gene targeting in embryonic stem cells for the generation of disease models and for the identification and validation of new drug targets. Within Dr Freichel's lab, transgenic reporter lines (e.g. GCaMP6) are crossed with disease carrying lines to enable visualization of impaired signaling processes. In parallel, electrophysiological measurements via patch pipettes are also carried out.
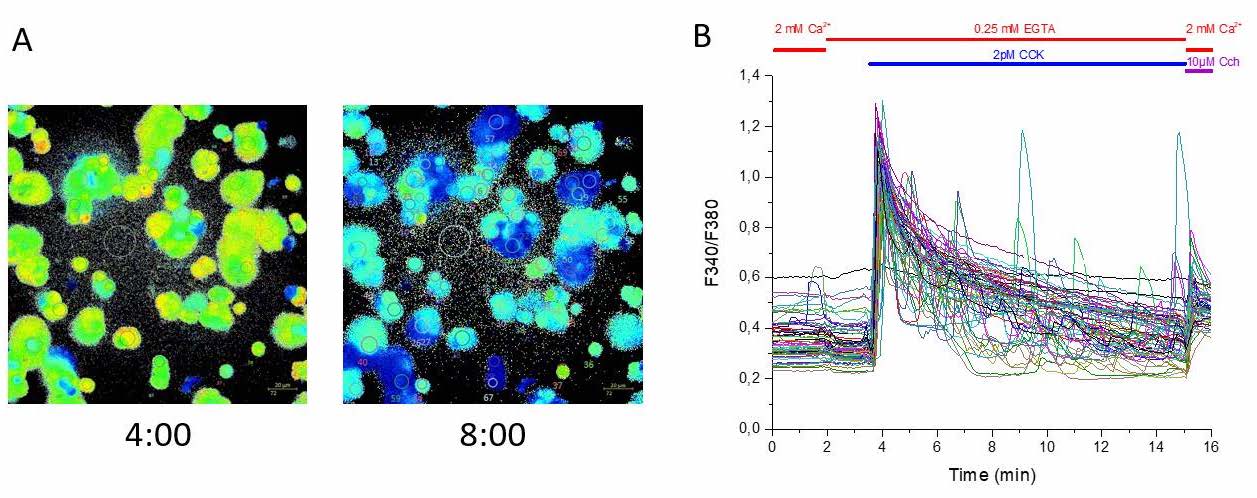
Figure 1. Time lapse of Fura-2 imaging in mouse pancreatic acinar cells. Cells were stimulated with 2pM cholecystokinin (CCK) in physiological salt solution (PSS) supplemented with 0.25mM EGTA following 10µM Carbachol (Cch) in PSS containing 2mM Calcium. Figure 1A visualizes intracellular calcium concentrations immediately after (time point 4:00 min) and 4 minutes after application of CCK (time point 8:00 min). Representative F340/F380 ratios of 65 cells in response to 2pM CCK and 10µM Cch can be observed in Figure 1B.
Challenge
As with all reporters, individual light dose per image needs to be kept at a minimum to reduce potential side effects caused by phototoxic by-products. Moreover, the studied networks of cells exhibit spontaneous Ca2+fluctuations which can be - dependent on cell type and disease model - very rare so many separate fields-of-view need to be imaged to reach a reliable statistical conclusion.
When transgenic reporters can't be used, the lab applies indicator dyes such as AM-esters of Fura2 and Fluo-variants. Here, a further problem can occur when too high dye concentrations need to be used to reach a detectable signal level. This surplus of dye alters the kinetics of the physiological response, rendering it a non-physiological situation. This problem can only be overcome by reducing the concentration of dye, which means a scientific camera is needed that is sensitive enough to pick up very low signals.
Their current imaging solutions are either state-of-the-art CCD sensors or EMCCDs. Both require a very long exposure time and provide images with a suboptimal signal-to-noise ratio. The small field of view of CCD-based sensors also requires many imaging sessions to acquire statistically significant data.
The field of view of the Prime 95B is >2.5x bigger than our previous camera, allowing us to image more than twice the amount of data in a single imaging session and the high sensitivity of the Prime 95B dramatically reduced the exposure time needed to collect our images.
Marc Freichel,Volodymyr Tsvilovskyy
Solution
The Photometrics Prime 95B back-illuminated sCMOSis the perfect match for the imaging demands of the Freichel lab.
The increased sensitivity achieved by the 11 µm pixel and the peak-quantum efficiency of 95% at a wavelength range which matters mostly for their reporters dramatically reduces the exposure time for individual images. This carries two benefits. Firstly, kinetics can be determined more accurately as more data points can be acquired in the same amount of time. Secondly, less light needs to be used to detect their cells which allows the group to obtain more physiologically relevant data.
The field of view of the Prime 95B is >2.5x bigger than their previous camera solution, allowing the group to image more than twice the amount of data in a single acquisition, further improving their statistics and their time spent at the microscope.
The Prime 95B is also fully supported in Zeiss' ZEN software which is convenient as the group is already familiar with the interface.
In brief, the Prime 95B enhances the Freichel lab's scientific output and opens up new ways for them to study their samples of interest, bringing them closer to finding answers to their scientific questions.

Learn More About The Prime 95B