Super Resolution Spinning Disk Confocal Microscopy
Introduction
Spinning disk confocal microscopy (SDCM) is a versatile and widely-used imaging technique in biology due to its ability to perform fast, 3D imaging of live cells. Recently, techniques have been created that combines the high resolution of super-resolution fluorescence microscopy with the simplicity and optical sectioning capability of SDCM, resulting in a spinning disk system capable of a 2x resolution improvement over the diffraction limit.
Confocal microscopy uses optical sectioning to take multiple thin, 2D slices of a sample that are used to construct a 3D image. This process removes the out-of-focus light from other planes, giving a high-contrast image which is often further processed to improve image quality. Compared to other optical sectioning techniques, SDCM is high-speed, high-sensitivity and simple to implement. However, the resolution is limited by the diffraction limit of light which typically prevents detailed imaging of structures below ~200 nm.
Super-resolution fluorescence microscopy allows for structures to be resolved beyond the diffraction limit, which is of substantial benefit to researchers investigating processes that occur at this level. There are many super-resolution techniques currently available (such as STED, SIM, STORM, and PALM) and they have different capabilities to suit different applications. However, the ability to increase spatial resolution with these advanced techniques comes at the cost of technical complexity, dye restrictions, and typically slower imaging speed.
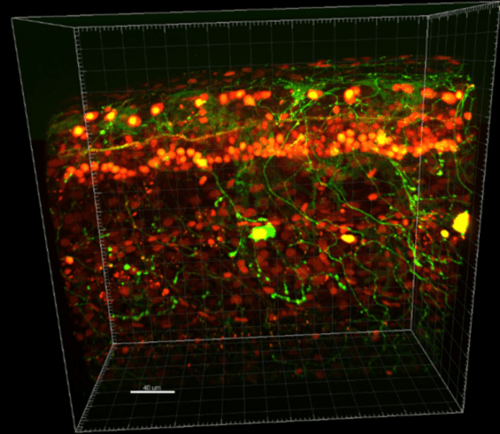
Figure 1: Imaging of zebrafish embryos using super-resolution spinning disk confocal microscopy using
the Prime BSI sCMOS. The image shows a single embryo labeled with nuclear (red) and neuronal (green)
markers, scale bar 40 μm. Image from Dr. Urs Ziegler Prime BSI Customer Story.
Combining super-resolution with SDCM overcomes many issues of both techniques to create a powerful new technique for live cell imaging that promises high sensitivity, resolution, and speed.
Super Resolution Confocal Theory
It has long been known that confocal microscopy could surpass the diffraction limit and reach super-resolution levels, as proposed by Sheppard (1988) and implemented by Mueller and Enderlein (2010) as imaging scanning microscopy (ISM).
Due to the way confocal microscopes are set up (excitation light focused through a pinhole, emitted light detected through a pinhole), there are two point spread functions (PSFs) in the system: the excitation illumination PSF at the sample plane and the emission detection PSF at the pinhole plane, as seen in Fig.2B. An image is generated from the combination of the illumination PSF and imaging PSF, so narrowing either PSF can provide increased resolution.
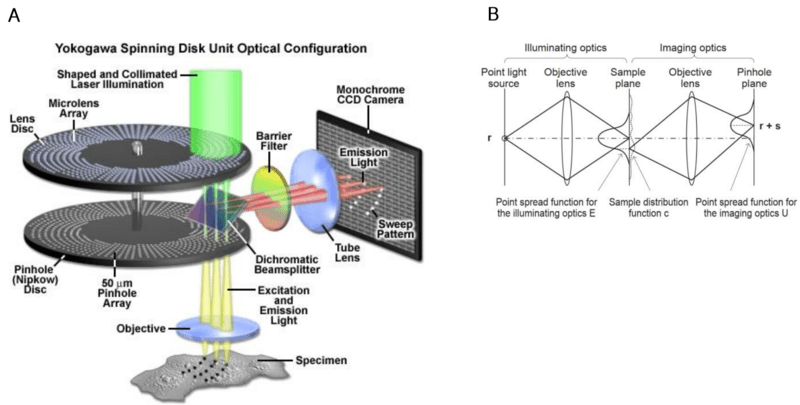
Figure 2: Illustration of a Yokogawa spinning disk system and PSFs. A) The top disk with microlenses focuses the laser light to the imaging pinhole of the bottom disk to increase light throughout. As the disks spin, imaging of the sample through the pinholes fills the field of view of the camera. B) Optical system. For simplicity, the illuminating/imaging pics are drawn separately. The PSF for the illuminating optics has its peak at r whereas the PSF for the imaging optics has its peak at r + s. A is adapted from Zeiss (intro to spinning disk), B is adapted from Azume and Kei (2017).
An image is generated from the combination of the illumination PSF and imaging PSF, but these two PSFs are offset (see Fig.2B and Fig.3). Narrowing either PSF would increase the resolution of the confocal microscope. The illumination PSF is diffraction-limited but there are options available to manipulate the imaging PSF. For example, the confocal-like super-resolution method stimulated emission depletion microscopy (STED) narrows the emission PSF by suppressing fluorescence emission of the central axis of the excitation PSF (Roobala et al. 2018).
In the same way, narrowing the pinhole of a confocal or focusing the imaging PSF can lead to an increased resolution in the image. Although the theoretical maximum resolution of a confocal microscope is √2x (1.4x) the diffraction limit, this is only when using an infinitely small pinhole, which is sadly impossible to manufacture in reality and would block far too much light. As an alternative to smaller pinholes and in order for confocal to surpass the diffraction limit, Sheppard and colleagues (Sheppard 1988, Sheppard et al. 2013) came up with a technique known as photon reassignment.
Photon Reassignment
The photon reassignment principle explains that it is possible to take the offset detected light and determine a more probable position for the emitted light. In essence, emitted photons detected from the excited sample are reassigned to the most probable locations that they originated from. This reassignment occurs for every single scan position, which results in an increased maximum resolution (Azuma and Kei, 2015).
As shown in Fig.2B and Fig.3, in a confocal microscope the illumination PSF peaks at r while the imaging PSF peaks at r +s, meaning that the PSFs do not perfectly overlap.
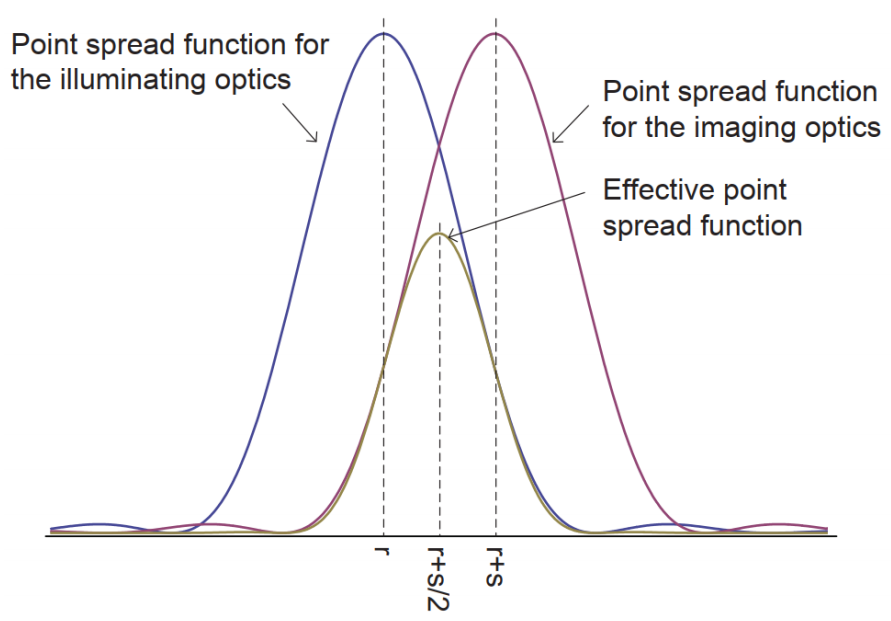
Figure 3: Effective PSF of a spinning disk confocal microscope. Adapted from Azume and Kei (2017).
The problem is that while a confocal microscope is imaging at the position r+s, the actual image is most likely coming from the position r+s/2, the point halfway between the two PSFs. This offset results in a reduction in spatial resolution.
Ideally, the imaging point should be shifted from r+s to r+s/2, which is the goal of photon reassignment. The image formed at r+s/2 has a narrower PSF, which results in an increased maximum resolution of √2x. This process makes the resolution nearly equal to an ideal confocal microscope, in which the pinhole has been reduced to an infinitely small size. Following this up with deconvolution would make it theoretically possible to get a 2x higher spatial resolution than the diffraction limit (Azuma and Kei, 2015).
This photon reassignment was originally done computationally but this slowed down image acquisition significantly. Instead, it was found to be possible to reassign photons optically with lenses, so that the detected light is already in the optimal position (rather than offset). The result is optical photon reassignment microscopy, and this was applied to other microscopy techniques including single-point confocal (Sheppard et al., 2013), array point scanning confocal (York et al., 2013), and spinning disk confocal systems (Shroff and York, US Patent awarded 2017).
Super Resolution Spinning Disk Confocal Microscopy
As seen in Fig.4, some SDCM systems feature a micro-lens disk above the primary pinhole disk in order to better focus the excitation light through the pinholes.
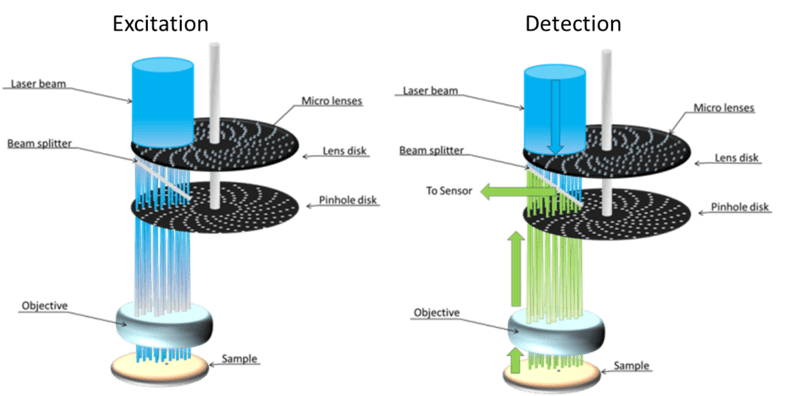
Figure 4: A SDCM featuring a micro-lens disk and a pinhole disk. During excitation, the laser is focused by
the micro-lenses through the pinholes onto the sample. Both discs spin synchronously so all the sample
can be excited. During detection, the emitted fluorescence from the sample passes through the pinholes
where it is detected by a camera.
These two-disk systems can be slightly modified to optically reassign photons in order to reach super‑resolution, through the simple addition of micro-lenses on the underside of the pinhole disk, as seen in Fig.5.
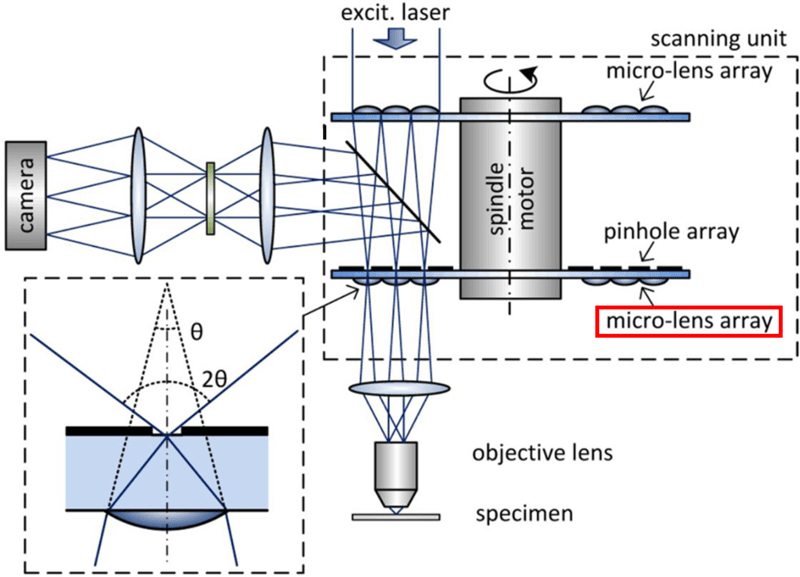
Figure 5: Optical path of a super-resolution SDCM. The key difference to a standard SDCM (seen in Fig.2 and Fig.4)
is the addition of micro-lenses to the underside of the pinhole array disk (highlighted by the red square). The bottom left
inset indicates how these new micro-lenses allows light coming from up to twice the angle to pass through the pinhole.
This increases the resolution through optical photon reassignment. Image adapted from (Azuma and Kei 2017).
Emitted light from the sample passes through these extra micro-lenses first (as seen by the red square in Fig.5), then the pinholes, and then to the detector. The extra micro-lenses essentially doubles the angle at which emitted light can pass through the pinhole from the sample (as seen in the insert in Fig.5, the convergence angle of light emitted from the sample is doubled from θ to 2θ), which halves the size of the focus, contracting the focus down to a much smaller point. This increased focus optically reassigns the emitted photons to where they most likely originated from and increases the maximum resolution by up to √2x, up to 2x after deconvolution. The result is a super-resolution spinning disk confocal microscope.
With this system, the speed and optical sectioning advantages of a SDCM are maintained but are now combined with super-resolution imaging. By rotating the disks at 4000 rpm, super-resolution images can be captured live at up to 200 fps using most fluorescent dyes, overcoming many of the drawbacks of conventional super-resolution techniques and combining the best of both techniques. This system is currently manufactured by Yokogawa as the SoRa (super-resolution by optical reassignment), and example images from such a system can be seen in Fig.6.
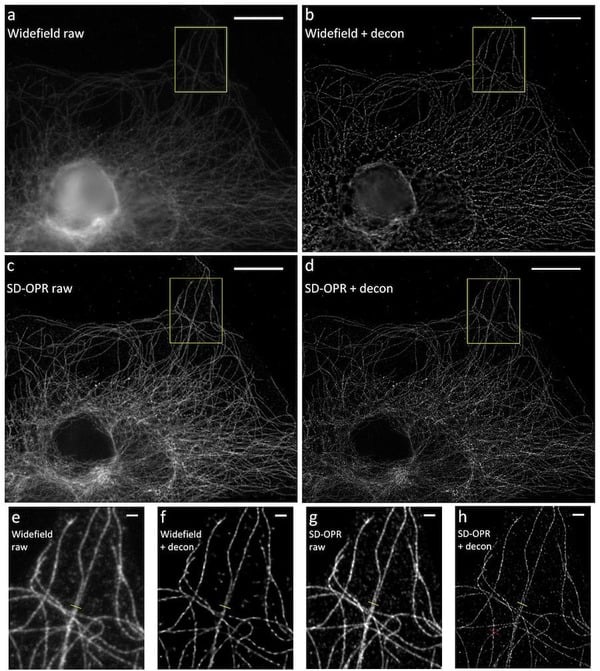
Figure 6: Comparison of images of microtubules. A) Standard widefield, B) widefield with deconvolution, C) spinning
disk optical photon reassignment (SD-OPR) and D) SD-OPR withdeconvolution. E-H represents the yellow square in the
corresponding images A-D. Deconvolved images were obtained by applying 3D deconvolution using Huygens. Scale
bars represent 10 µm. Adapted from Azuma and Kei (2015).
This results in a SDCM that has a maximum resolution limit of ~120 nm (as opposed to the diffraction-limited SDCM maximum of ~250 nm). Due to this high resolution, it is necessary to pair these systems with a camera that is also capable of sampling at super resolution levels. It is vital to use an appropriate scientific camera for super resolution SDCM with the pixels optimized for Nyquist sampling at higher magnifications, as this kind of system uses very high magnifications, often over 200x by combining objectives.
Cameras for Super Resolution Spinning Disk
Cameras that maximize sensitivity and speed are typically used for super resolution SDCM, which means that back-illumination CMOS cameras are generally preferred.
Typical cameras used with these systems have either a 6.5 μm or 11 μm pixel size, to be used with 240x or 280x magnification respectively. This allows for Nyquist sampling at 62 nm and 90 nm, either of which is suitable for oversampling from 120 nm. The smaller pixel size of a 6.5 μm camera may give slightly improved resolution but at the cost of reduced sensitivity, detecting 2.2x fewer photons than an 11 μm pixel camera. On the other hand, an 11 μm pixel camera greatly improves contrast and allows for exposure times to be reduced while still oversampling enough for effective deconvolution.
Summary
Super resolution spinning disk confocal microscopy represents a highly successful marriage between two complex techniques that is greater than the sum of its parts. Optical contraction of the imaging PSF provides a mechanism by which resolution can be increased beyond the diffraction limit. The Yokogawa SoRa is currently the most widely used super resolution SDCM, providing an increase in resolution through optical photon reassignment via a microlens array on the underside of the pinhole disk. By allowing confocal microscopes to increase resolution beyond the diffraction limit of light, powerful images of live biological samples can be taken with high sensitivity, super-resolution, and high speed. This technique is capable of reliable super high resolution observations in biomedical routine research and is a vital part of advanced microscopy.
References
Azuma, T. and Kei, T. (2015) Super-resolution spinning-disk confocal microscopy using optical photon reassignment. Opt Express. Jun 1;23(11):15003-11. doi: 10.1364/OE.23.015003.
Azuma, T. and Kei, T. (2017) Development of high-speed super-resolution confocal scanner. Yokogawa technical report English Edition. Vol 60(2) 33-37.
Muller C.B. and Enderlein J. (2010) Image scanning microscopy. Phys Rev Lett, 104:19
Roobala C., Illanila I.P. and Basu J.K. (2018) Applications of STED fluorescence nanoscopy in unraveling nanoscale structure and dynamics of biological systems. J Biosci., 43 (3): 471-484
Sheppard, C. J. R. (1988) Super-resolution in confocal imaging. Optik (Stuttg.) 80(2), 53-54.
Sheppard, C. J. R., Mehta, S. B. and Heintzmann, R. (2013) Superresolution by image scanning microscopy using pixel reassignment. Optics Letters Vol. 38, No. 15 2889
Shroff, H and York A. 2017 US Patent #9696534 Multi-focal structured illumination microscopy systems and methods
York, A. G., Chandris, P., Nogare, D. D., Head, J., Wawrzusin, P., Fischer, R. S., Chitnis, A., Shroff, H. (2013a) Instant super-resolution imaging in live cells and embryos via analog image processing. Nat Methods. Nov;10(11):1122-6
http://zeiss-campus.magnet.fsu.edu/articles/spinningdisk/introduction.html
https://www.visitron.de/products/visiscope-confocal/yokogawa-sora.html
Acknowledgments
We would like to thank Hari Shroff for comments on the manuscript.
Download As PDF
Further Reading
Back To Spinning Disk
Join Knowledge and Learning Hub