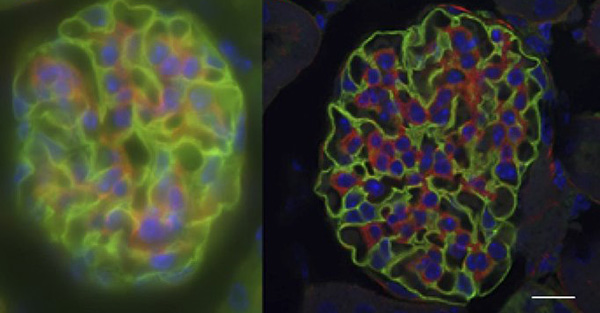
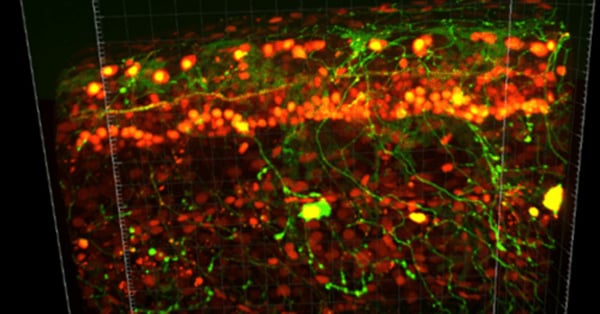
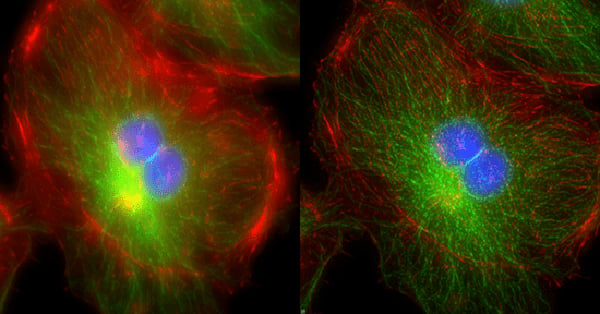
Confocal microscopy addresses two significant challenges in biological imaging that conventional fluorescence microscopy cannot overcome. Firstly, biological specimens are 3-dimensional structures so to fully understand them we often need to construct 3-dimensional images. Secondly, many processes biologists would want to study occur inside biological structures, but other cell features can block a clear view.
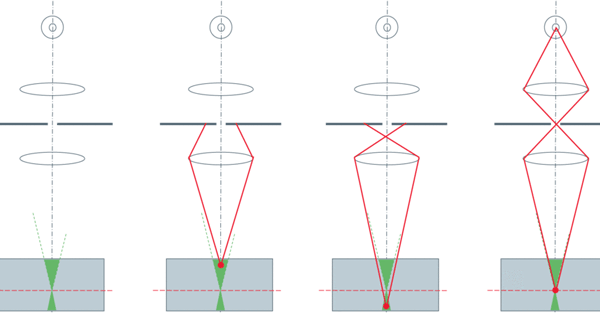
Confocal microscopy uses optical sectioning to take multiple, thin, 2-dimensional slices of a sample to construct a 3-dimensional model from them. This is made possible through the addition of a pinhole into the same focal plane as the sample to block out-of-focus light. Spinning disk confocal microscopy increases the speed of this technique by using multiple pinholes etched into an opaque disk which, when spun, scans the pinholes across the entire image.
Spinning disk confocal microscopy is, essentially, a light rejection technique so one of the main challenges is to collect as many of the emitted photons as possible so light intensity can be reduced to lessen the impact of photobleaching and photodamage on samples, which means that high camera sensitivity is of critical importance.