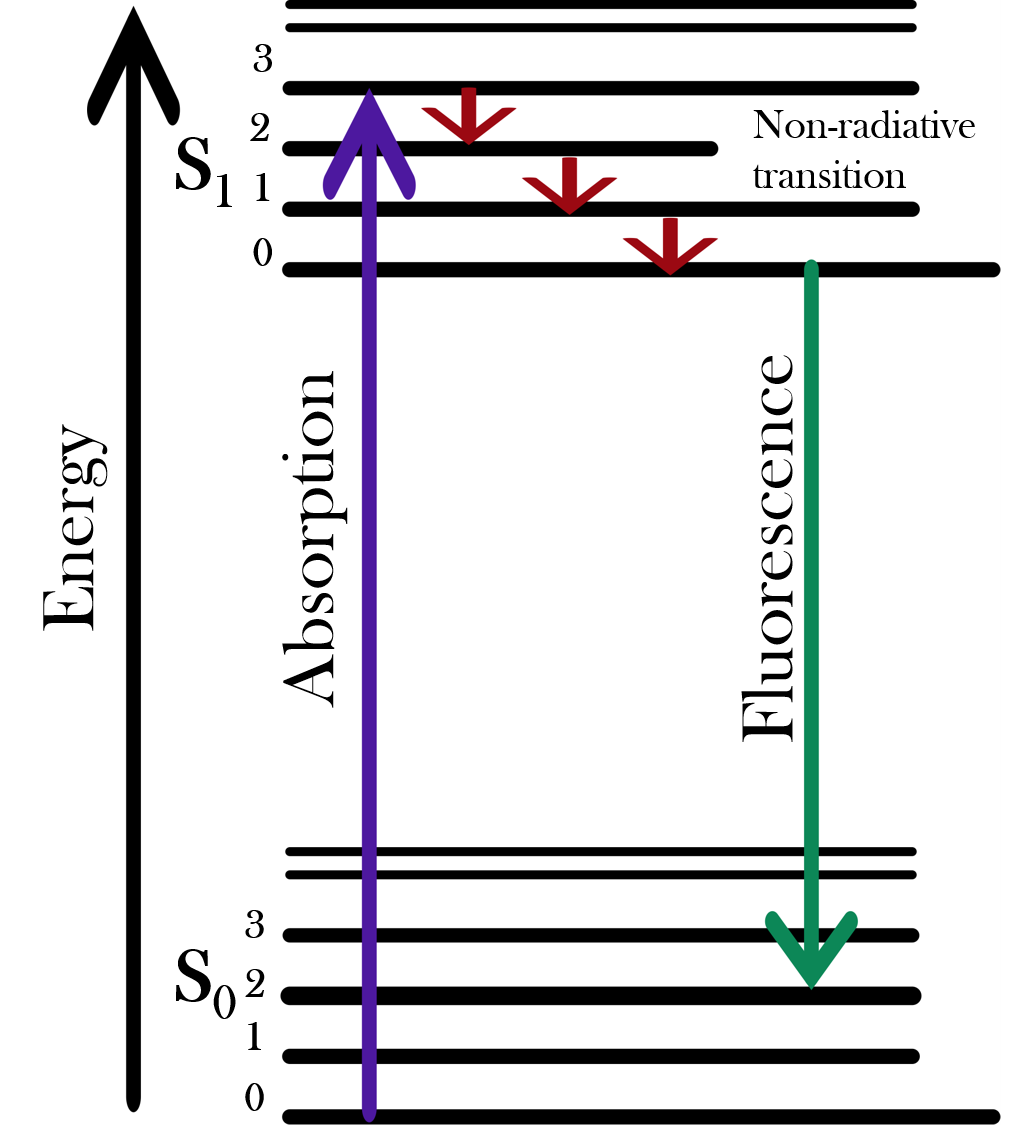
Fluorescence, phosphorescence, and photoluminescence occur when a sample is excited by absorbing photons and then these photons are emitted with a characteristic decay time. Fluorescence is when the absorbing and emitting species is an atom or molecule. Phosphorescence is similar to fluorescence but with a longer time gap between absorption and emission. Photoluminescence is the term physicists use to describe the absorption and emission of light by materials such as semiconductor and nanotubes.
A chemical species absorbs a photon and is excited to a singlet electronic state. It then relaxes via non-radiative mechanisms and emits a lower-energy photon. This allows it to transition to the ground electronic state. A spectrograph and camera can be used to obtain the spectrum of emission, with the intensity correlating to the concentration of the chemical species.