Introduction to X-ray Scattering
Introduction
When a sample is illuminated by x-rays, these incident x-rays can be deflected and scattered by the sample, producing complex patterns. Analysis of these patterns, their intensities as well as the angle of scatter (incident vs scattered x-rays), changes in polarization, wavelength, and/or energy, can reveal structural, elemental and atomic information about the sample, and are known as x-ray scattering techniques.
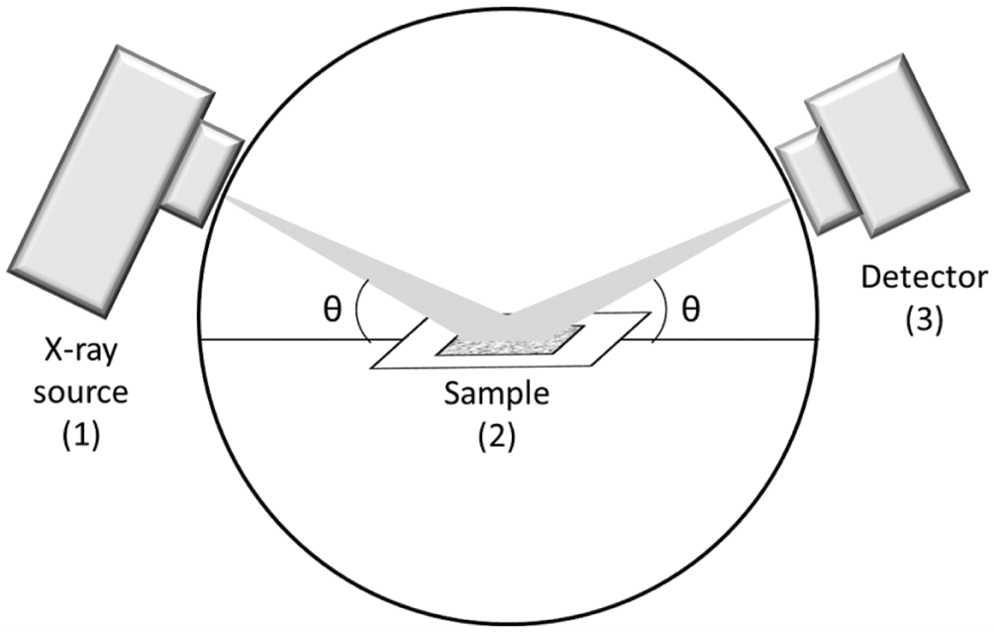
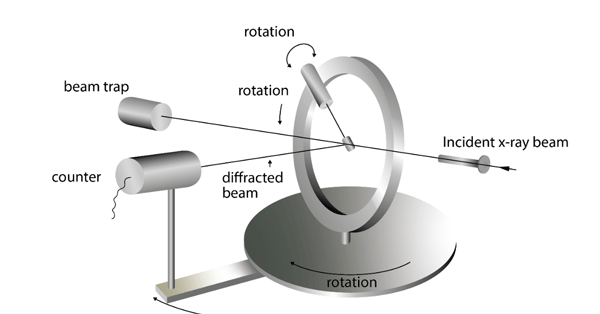
Figure 1: X-ray scattering. An x-ray source (1) sends incident x-rays to the sample
(2) at an incident angle, at which point a proportion of the incident x-rays are
scattered from the sample at a similar or different angle, to the x-ray detector (3).
X-ray scattering can be applied to a wide range of different sample types, from simple repeating crystals to novel materials and complex biological molecules/polymers. These techniques are non-destructive and are commonly used to probe samples alongside x-ray microscopy and x-ray spectroscopy. If samples can scatter x-rays, they can be analysed with x-ray scattering. Sample size, general shape, dispersity (distribution of size and shape of molecules within a sample), porosity, morphology, orientation and more can all be measured with x-ray scattering.
The x-rays are scattered by electrons within the sample, this means the more electrons, the more scattering, meaning that heavier elements with many electrons are often highly suited for scattering experiments. Another consideration is contrast, in this case contrast works based on differences in electron density within a sample. A desirable sample would be highly electron-dense, and surrounded by a sparse medium, so there will be significant contrast and the sample can be easily analysed. Matrices with a similar electron density to the sample should be avoided, such as imaging silicon particles in a silica gel, many scattering experiments can submerge samples in solution to enhance contrast.
The scattered x-rays from electrons within a sample will interfere, in electron dense areas of the sample, meaning that detectors are typically analysing and characterising scattering that has undergone constructive interference within the sample, allowing for identification of electron location and other structural information.
Detectors will output a 2D scattering pattern, with the beamstop in the centre followed by scattering, this can be used to generate a 1D scattering curve that can be fit to certain samples. This can be used to infer size, shape and surface features of samples. Powders and dispersions will have more random scattering patterns, when compared to more structured samples such as fibres, and with crystals having highly anisotropic, separate scattering patterns (diffraction-like scattering).
X-ray Scattering Techniques
X-ray scattering techniques can be broadly divided into elastic or inelastic scattering. With elastic scattering, the scattered x-rays have the same energy/wavelength as the incident x-rays, whereas with inelastic scattering, the scattered x-rays have a different energy/wavelength.
One of the most well-known elastic scattering techniques is x-ray diffraction/crystallography, these techniques are both subsets of x-ray scattering that use crystalline samples and require very high energy hard x-rays (with an extremely small wavelength) to discern details at the atomic level. This document will focus on other techniques in the x-ray scattering category, such as the additional elastic scattering techniques small-angle x-ray scattering (SAXS) and wide-angle x-ray scattering (WAXS).
Inelastic techniques include Raman scattering and resonant inelastic x-ray scattering (RIXS), both of which are covered in a x-ray spectroscopy document.
Small-angle x-ray scattering (SAXS) and wide-angle x-ray scattering (WAXS)
X-rays can be elastically scattered at a variety of different angles by different samples, by looking at the angle of scattered x-rays, different sample information can be obtained. SAXS analyses elastic x-ray scattering at very small angles, typically 0.1-10°, while WAXS makes use of wider angles, typically >10°.
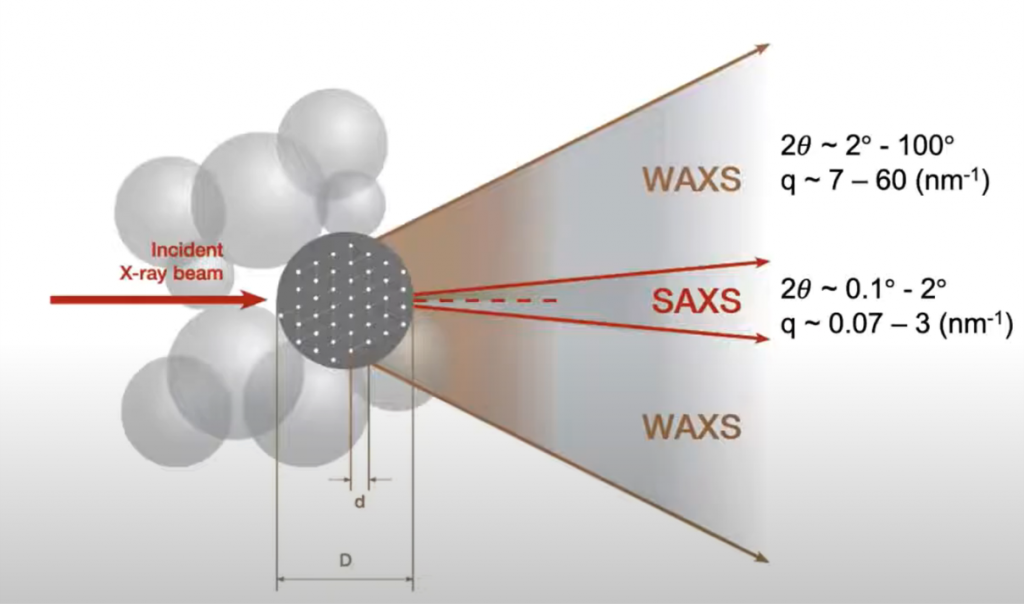
Figure 2: SAXS and WAXS achieve different resolutions, with WAXS measuring wider
angles, and achieving atomic resolution, and SAXS measuring very small angles and
achieving nanoscale resolution.
By detecting x-rays scattered at different angles, different sample information is gained at different resolutions: SAXS has nanoscale resolution (good for samples 100-1 nm in size) while WAXS has atomic resolution (1-0.1 nm). Due to this, SAXS can be typically used to analyse larger scale bulk microstructure within a sample, while WAXS is more similar to x-ray diffraction and can observe atomic details. SAXS and WAXS can be done simultaneously, and easy interchanged, as it simply involves moving the detector and sample closer or further away from each other.
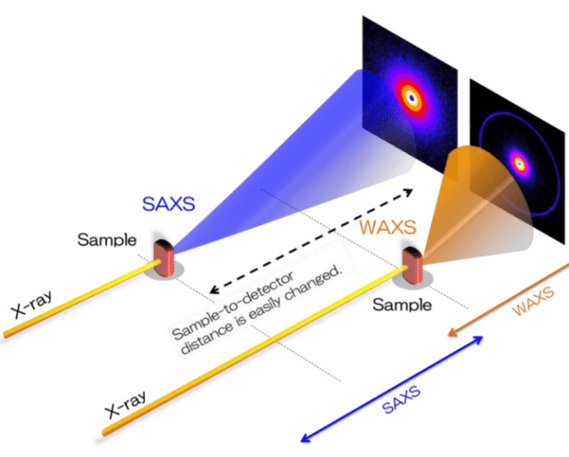
Figure 3: SAXS and WAXS can be done simultaneously by moving the detector closer or further
away from the sample.
As SAXS/WAXS can be used on non-crystalline samples, they can gather information about highly complex samples, including proteins and composite systems, and have been used to study drug delivery systems, formulations, phase behaviour, as well as advanced materials development.