NIR-II Probes for In vivo Imaging
Introduction
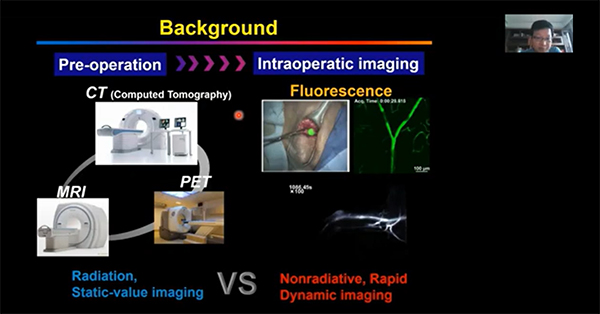
Optical fluorescence imaging is one of the most common techniques for imaging in vivo, due to its high temporal and spatial resolution [1]. As it is a non-invasive, real-time technique it is an attractive imaging modality for medical applications such as cancer diagnostics, biosensing, and medical testing.
The majority of fluorescence probes, essential for optical fluorescence imaging, reside within the visible range (400-700 nm). However, visible light is limited when imaging within the body. Active biological components, such as haemoglobin and water, absorb light within the visible range, increasing their opacity. These components also cause visible light to scatter within biological tissue, reducing the penetration depth of visible fluorophores. In addition, biological tissues also self-fluoresce (called autofluorescence), within the visible range as they contain multiple luminescent macromolecules which influence any image acquired.
Near-infrared (NIR) light is less absorbed by biological components within tissue, thus reducing light scattering and autofluorescence. Therefore, development into the creation of fluorescent probes that emit within the NIR has increased. The first NIR optical window (NIR-I), encompasses wavelengths within the 700- 950 nm range. Tissue components have much lowed attenuation coefficients within this range, contributing to a deeper penetration depth. However, NIR-I is limited for in vivo imaging as tissue autofluorescence is still prevalent, allowing only for a penetration depth of 1-2 cm [1-3].
NIR-II is the second NIR optical window and encompasses the wavelength range 1000 - 1700 nm. Within this range the attenuation coefficient of biological components decreases further still, reducing the opacity of tissue and the amount of light that scatters within the tissue. The NIR-II wavelength range also reduces autofluorescence of biological tissue, owing to a deeper penetration depth and increased image quality in comparison to visible light and NIR-I [1-4]. NIR-II fluorescent probes are still in the early stages of development, with research hindered by availability of probe material and cost of the imaging equipment. Yet research has shown promise for the use of NIR-II fluorophores as therapeutics, contrast agents, and biomarkers amongst many other applications [1]. Within this application note, the range of commonly used NIR-II fluorescent probes will be discussed, alongside clinically approved NIR-II probes and future research focus.
Silver Chalcogenide Quantum Dots
Quantum dots (QDs) are small nanocrystals (1-10 nm) with fluorescent properties. Generally considered to be zero-dimensional semiconductors, the size of QDs can be finely tuned to emit over a wide range of wavelengths, from UV to IR. These nanomaterials have been investigated as NIR fluorescent probes due to the narrow band gap between the valence and conduction bands of the QDs (Figure 1), allowing them to be excited by NIR-I wavelengths, while emitting in the NIR-II window [2]. The energies of this band gap decrease with an increase in QD diameter, allowing for complete control over the desired absorption and fluorescence.
Although there are numerous NIR-II fluorescent QDs, the majority contain heavy metals, owing to high bio-toxicity inappropriate for clinical use. However, silver chalcogenide QDs (Ag2X where X = S, Se and Te) are reportedly non-toxic, with a narrow band gap, making them ideal NIR-II fluorescence probes. These QDs can be tuned to fluoresce over the 510-1330 nm range, covering both the NIR-I and NIR-II windows [1,2]. Ag2X QD surfaces can also be functionalized, with specific targeting moieties conjugated to ensure the QDs accumulate within the tissue of interest [2].
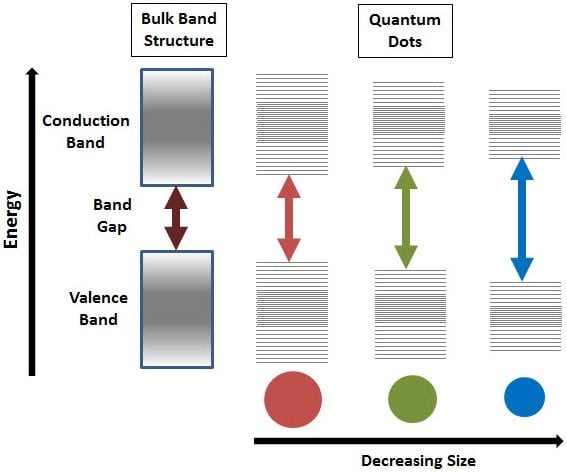
Figure 1: Schematic showing how the difference in quantum dot size relates
to the difference in band gap, with a decreasing size increasing the band gap
width. This increase corresponds to a blue shift in wavelength emission. Image
obtained from [5].
Ag2X QDs have both high biocompatibility and high photostability, with photostability 100-1000x higher than that of common organic dyes and 100x more fluorescence. These QDs are not only able to visualize deeper structures, but also have high spatiotemporal resolution, achieving spatial resolution down to ~24 μm and temporal resolution of 50 ms. As they have high photostability, they are ideal probes for long exposure applications, such as image-guided surgery and drug delivery [2].
Long exposure experiments can require several minutes to acquire data, increasing not only the detected signal but also any associated noise. InGaAs sensors are dark-noise-limited due to the low bandgap of InGaAs material, meaning that deep-cooling is essential to reduce noise and maintain appropriate signal-to-noise ratios for scientific applications. Two common ways to cool InGaAs cameras are thermoelectric cooling (TEC) and cryogenic cooling. TEC cooling is advantageous as it is a maintenance-free cooling method which is able to sufficiently reduce noise for scientific imaging without compromising imaging speed. However, TEC is not able to cool the system to as low a temperature as cryogenic cooling, meaning that it is unsuitable for ultra-low signal detection. The following graph demonstrates the ability to reduce dark noise through deep cooling.
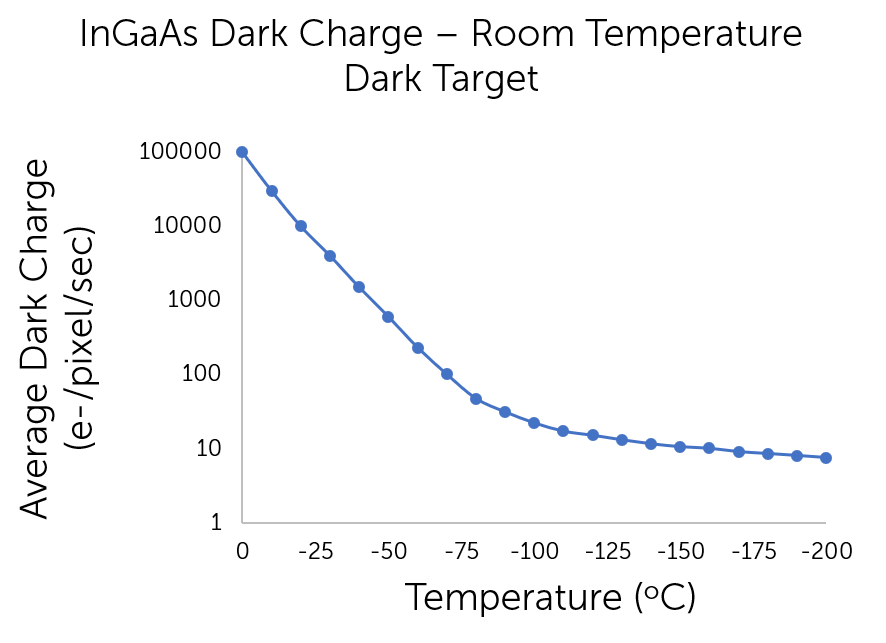
Figure 2: The relationship between dark noise with respect to temperature, indicating a reduction in
electrons per pixel per second with a reduction in temperature.
Single Walled Carbon Nanotubes (SWCNTs)
SWCNTs are quasi-one-dimensional semiconductors (Figure 3) that behave similarly to QDs, with a narrow band gap attributing to their use as NIR fluorescent probes [1,2]. By changing their size, SWCNTs can fluoresce over the 1000-1700 nm range, encompassing the entirety of the NIR-II window [2]. SWCNTs have low cytotoxicity while remaining highly photostable, allowing them to be used in applications such as image-guided surgery, photothermal therapy, cancer diagnostics, vascular imaging, and drug delivery [1,2]. SWCNTs also remain in the blood for a long time, allowing for long exposure in vivo studies [2].
Figure 3: Typical representation of a quasi-one-dimensional
single walled carbon nanotube.
SWCNTs were the first fluorescent probes investigated for NIR-II in vivo imaging, initially used to image vascular structure, providing a spatial resolution of ~10 μm and a high temporal resolution of <300 ms [1,2]. One of the main advantages of SWCNTs as fluorescent probes is that they have a large surface area. This means that multiple therapeutic medicines, genes, and ligands can be functionalized onto the surface to provide targeted treatment [6]. However, fluorescence of SWCNTs is highly dependent on the chemical environment, with SWCNTs usually having a low fluorescence quantum yield of <1% [1,2]. Therefore, any functionalization changes must try to maximize NIR-II fluorescence.
As SWCNTs have low fluorescence yield, they require an InGaAs sensor with high sensitivity and low noise. Although TEC cooling for InGaAs sensors is advantageous due to its design, SWCNT imaging may require lower temperatures to minimize noise while detecting faint NIR-II fluorescent signal. Therefore, cryogenically cooled InGaAs cameras are ideal to obtain maximum signal intensity while reducing the level of dark noise, especially for SWCNT long exposure experiments. Figure 4 is an in vivo image captured with a 640 x 480 InGaAs camera cooled to -190oC.
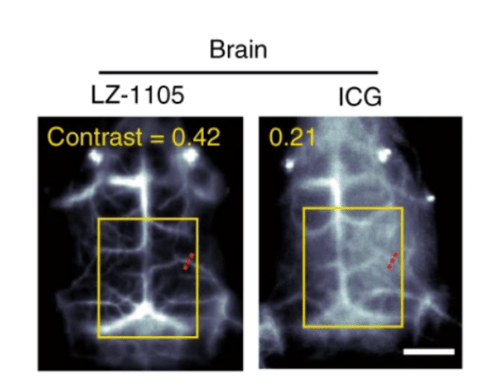
and ICG (NIR fluorescent dye) within a mouse brain. Image acquired using
a cryogenically cooled InGaAs camera [7].
Conjugated Polymer Nanoparticles
Conjugated polymer nanoparticles (CPNPs) are semiconductors comprised of copolymerized polymers with large π-conjugated backbones [1,8]. CPNPs are useful biological fluorophores as they are highly photostable (allowing for long exposure times), hydrophobic (so are readily accepted by cells), and have low toxicity [8]. They can also be functionalized with biomolecules to specifically target biological tissues of interest, making CPNPs a highly specialized fluorophore [2,8]. CPNP fluorescence emission can be altered by changing the structures of the donor and acceptor regions within the polymer. This results in changes to the band gap energies of the polymer, allowing CPNPs to be used as tunable NIR-II fluorophores [1,8]. Schematics of the common synthesized variants of CPNPs are shown in Figure 5.
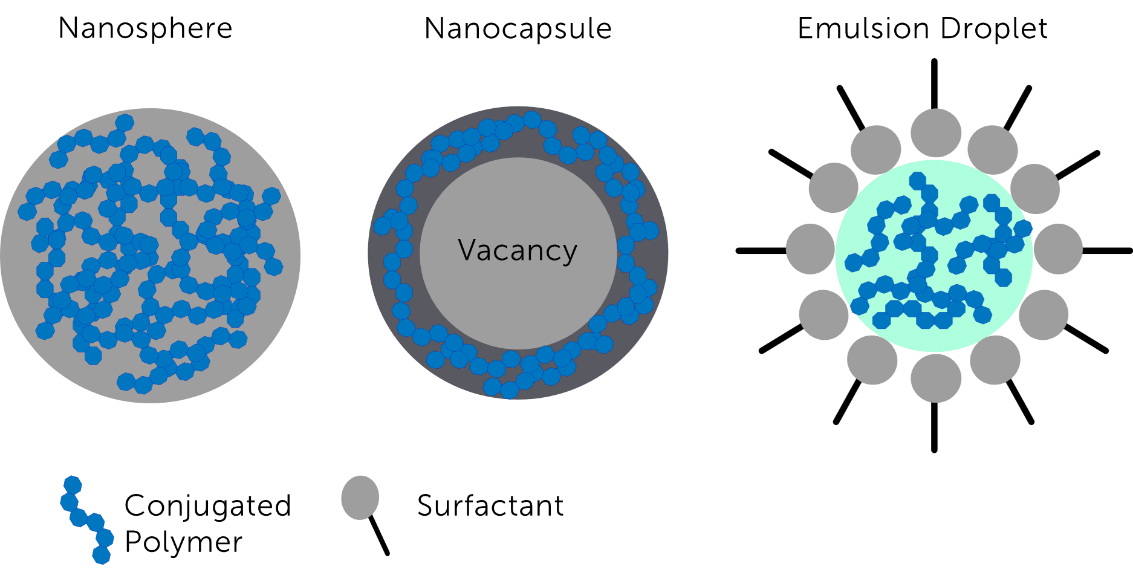
Figure 5: Schematic showing the different variations of conjugated polymer nanoparticles dependent on fabrication methods.
Image adapted from [9].
CPNPs are also used for long exposure applications, so therefore require similar camera technology to Ag2X QDs. PEG-capped CPNPs have been developed which have an enhanced quantum yield, in comparison to SWCNTs, and have shorter exposure time. This means that they can be used for real-time, dynamic NIR-II experiments [2]. These experiments would require a faster frame rate, as well as deep cooling. A high frame rate is achievable with TEC cooling, optimal for the high intensity, high speed, PEG-capped CPNPs.
The synthesis of CPNPs results in particles ranging from 40 - 500 nm, with post-polymerization techniques creating nanoparticles with diameters < 50 nm. However, for in-vivo applications, particles need to optimally be between 5 - 6 nm to be passed through the renal system. Anything larger than 6 nm needs to be broken down within the body before it can pass through the system. To achieve high fluorescence CPNPs need π-conjugated backbones, however, these cannot be broken down into smaller segments and are therefore cannot pass through an in-vivo renal system. This has prevented CPNPs from being used clinically, although they can still be used to provide useful biological information in-vitro [8].
Rare Earth Nanoparticles
Rare earth nanoparticles (NPs) that fluoresce within the NIR-II region are lanthanide-based NPs (such as NaYF4 or NaGdF4) that have been doped with other lanthanide metal ions [1,2]. As these rare earth metal ions have very narrow emission spectra over the wavelength range 1064 - 1310 nm, the NPs can be finely tuned to emit at the desired NIR-II wavelengths, as shown in Figure 6A. The rare earth metal ions can also be excited by two NIR-I wavelengths (808 nm and 980 nm), meaning that multicolor NIR-I-to-NIR-II fluorescence can be achieved from two different rare earth NP compositions simultaneously in-vivo [1].
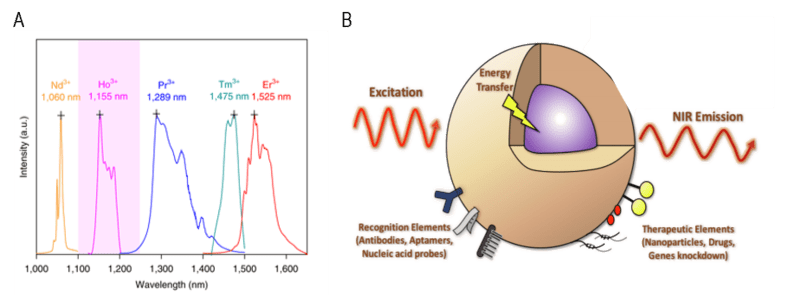
Figure 6: (A) Narrow emission bands over the wavelength range 1000-1700 nm relating to varying lanthanide-
based nanoparticles. Image obtained from [10]. (B) Schematic showing the general form of a core/shell
rare earth NPs with excitation light being absorbed by the NP, leading to downconverted NIR emission. Image
adapted from [11].
Lanthanide-based rare earth NPs that emit in the NIR-II region are downconversion nanoparticles [12]. Downconversion nanoparticles are able to absorb two or more photons with a short wavelength, convert these photons into a single, long wavelength photon, and then subsequently emit that longer wavelength photon [11]. This process requires a two-ion system in which a sensitizer (singly excited ion) transfers energy to an activator (photon emitting ion). The transition states which influence the fluorescence of rare earth NPs are mostly localized to the sensitizer and activator species [13].
This means that although the physical size of the NPs is not important, the size distribution needs to remain minimal. The concentration of sensitizer and activator ions is also essential to achieve optimal fluorescence from these NPs. By creating a core-shell structure, downconversion NIR emission can be precisely controlled on the nanoscale [2,12]. A depiction of this structure can be seen in Figure 6B. Downconversion NPs are advantageous as they provide narrow emission bands, less light scattering, and a larger anti-stokes shift [11].
Rare earth NPs have good biocompatibility and are predominately used for cancer detection as they accumulate well at tumor sites. This increased accumulation allows for long exposure, in-vivo tumor imaging, which enhances image quality as more fluorescence will be detected by the sensor [1]. Long exposure in-vivo imaging, over the NIR-II range, will require an InGaAs sensor that has ultra-low noise. Therefore, camera technology similar to Ag2X QDs and CPNPs is required, with ultra-long exposure imaging requiring cryogenically cooled InGaAs cameras.
The biggest limitation of rare earth NPs is that, although they have good biocompatibility and quantum yield, they rely on toxic, heavy metals and therefore cannot be used clinically. However, nanoparticle alloys have been developed that are able to maintain the biocompatibility and fluorescence properties of rare earth NPs but use non-toxic elements such as gold and copper [2].
Indocyanine Green Organic Fluorescent Dye
Although all of the previous NIR-II fluorescent probes mentioned within this document have shown great promise in in-vitro and preclinical studies, they have not yet been clinically approved. Indocyanine green (ICG) is an NIR organic fluorescent dye that has been approved by the Food and Drug Administration (FDA) for over 50 years [1,14]. It is commonly used in clinic for multiple applications such as tumor imaging, real-time surgical cardiac output, and non-invasive vascular imaging, with a penetration depth of <1 cm. ICG is excited between 740 - 800 nm, with an emission wavelength over the range of 800 - 860 nm (NIR-I). However, it has been discovered that ICG also emits over the 900 - 1150 nm range (NIR-II), with peak emission at approximately 900 nm, as shown in Figure 7. Therefore, ICG also has significant NIR-II fluorescence, with the added advantage of twice the contrast-to-noise ratios of ICG NIR-I fluorescence [1,14].
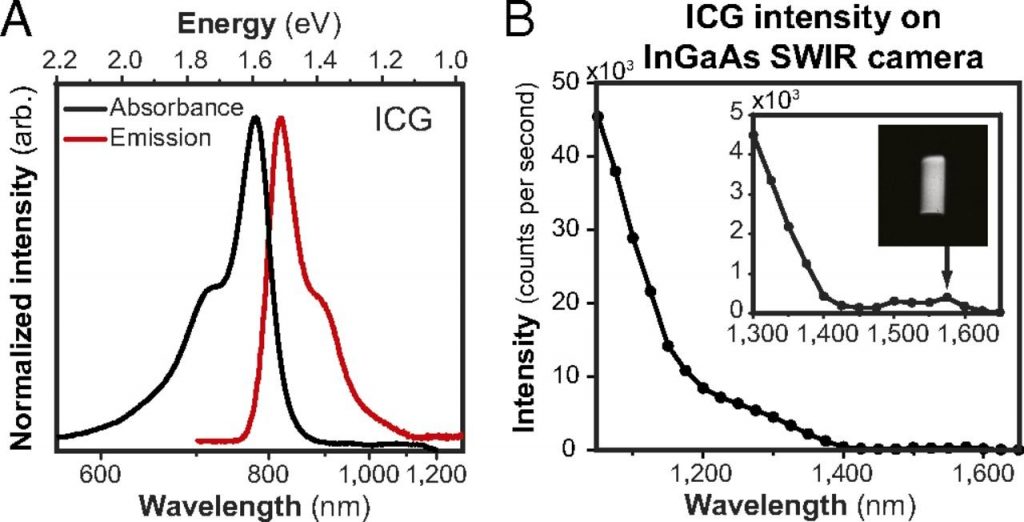
using an InGaAs camera, showing emission shoulder at approximately 900 nm. (B)
Emission intensity of a 0.027 mgmL-1 ICG solution (aqueous), showing emission up
to 1575 nm. Image obtained from [15].
Clinically approved NIR-II fluorophores have been predominately limited by the lack of sensitive camera technology. However, improvements in InGaAs sensor technology, essential for NIR-II detection, have provided an affordable solution. ICG is already FDA approved, meaning that there are strict guidelines regarding maximum dosage within patients. Although this maximum dosage provides sufficient NIR-I fluorescence, it was initially unclear if this concentration would also provide sufficient NIR-II fluorescence, especially as the NIR-II ICG intensity is lower than the NIR-I intensity. It was found that ICG NIR-II fluorescence was sufficient at clinical dosage, allowing for real-time, high frame rate imaging of vascular structures [15].
Although optimal for high speed imaging, organic NIR dyes such as ICG have low photostability. This means that while they can be used for dynamic imaging, they are not appropriate for long-exposure imaging [1]. High speed imaging requires a high frame rate InGaAs camera. TEC cooling of high frame rate InGaAs cameras results in dynamic NIR-II fluorescent acquisition at cool enough temperatures for low dark noise.
Another limitation of ICG is that it cannot be altered to target a specific location or tissue type due to a lack of functional groups. This prevents covalent conjugation of targeting molecules, rendering ICG dye a non-targeted probe. Yet, specific administration of the dye close to the target tissue provides ICG accumulation at the site of interest [16].
Conclusion and Future Research
The field of NIR-II fluorophores is very much active, with current research focussing on optimizing all types of fluorescent probes. Current research on small molecule-based fluorophores is concentrated on improving on current probes, for example optimizing fabrication design to improve fluorescent intensity [17] or altering response capabilities to certain wound cleaning chemicals [18].
A predominant limitation of small molecule-based fluorophores is a low quantum yield. Therefore, improving yield to ~14.8% using fluorophore rotor twisting and aggregation-induced emission, has been a research priority [19,20]. Other research has focused on improving yield and biocompatibility through newly fabricated small molecules, instead of modifying already existing NIR-II fluorophores [21,22]. As NIR-II fluorescent probes provide higher spatial resolution and deeper penetration, development into probes that emit at even longer wavelengths is being studied. For example, NIR-II fluorophores emitting at wavelengths of 1550 nm and above are now being fabricated for biomedical imaging [23].
Still, the biggest limitation for small molecule-based NIR-II fluorophores is FDA approval. Yet, since the discovery that ICG, a clinically approved organic dye, fluoresces in the NIR-II, many investigations to the efficiency of ICG for NIR-II in vivo imaging have begun. Recent studies show that ICG is being used within preclinical and clinical studies to, for example, determine vascular structures within the brain [24], for in-human image-guided surgery [25], and to visualize any bile tract injury post-surgery [26].
It is clear that NIR-II fluorophores are greatly enhancing in vivo optical studies, offering deeper penetration depth and higher resolution than visible light and NIR-I light-based fluorophores. Research has shown that there are multiple, highly-tunable small molecule-based fluorophores which allow for accurate control over both emission wavelengths in the NIR-II range and tissue targeting. These fluorophores are able to be used for both high speed, dynamic measurements and long exposure investigations, making them a useful tool in many clinical applications.
References
- Zhao J., Zhong, D., Zhou, S., NIR-I-to-NIR-II fluorescent nanomaterials for biomedical imaging and cancer therapy, Journal of Materials Chemistry B, 3, 2018
- Cao J., et al., Recent Progress in NIR-II Contrast Agent for Biological Imaging, Frontiers in Bioengineering and Biotechnology, 2020
- Bhavane R., et al., NIR-II fluorescence imaging using indocyanine green nanoparticles, Scientific Reports, 8, 2018
- Li Y., et al., NIR-II Fluorescence Imaging of Skin Avulsion and Necrosis, Frontiers in Chemistry, 2019
- Quantum Dots, Merck, https://www.sigmaaldrich.com/technical-documents/articles/materials-science/nanomaterials/quantum-dots.html#ref, accessed on 06.10.2020
- Diao S., et al., Chirality Enriched (12,1) and (11,3) Single-Walled Carbon Nanotubes for Biological Imaging, Journal of the American Chemical Society, 134, 2012
- Li, B., Organic NIR-II molecule with long blood half-life for in vivo dynamic vascular imaging, Nature Communications,11, 2020
- Repenko T., et al., Bio-degradable highly fluorescent conjugated polymer nanoparticles for bio-medical imaging applications, Nature Communications, 8, 2017
- Crucho C.I.C, Barros, M.T., Polymeric nanoparticles: A study on the preparation variables and characterization methods, Materials Science and Engineering: C, 80, 2017
- Fan Y., Lifetime-engineered NIR-II nanoparticles unlock multiplexed in vivo imaging, Nature Nanotechnology, 13, 2018
- Loo J.F-C., et al., Upconversion and downconversion nanoparticles for biophotonics and nanomedicine, Coordination Chemistry Reviews, 400, 2019
- Chen X., et al., Size-dependent downconversion near-infrared emission of NaYF4:Yb3+, Er3+ nanoparticles, Journal of Materials Chemistry C, 9, 2017
- Bouzigues C., Gacoin T., Alexandrou A., Biological Applications of Rare-Earth Based Nanoparticles, ACS Nano, 5, 2011
- Starosolski Z., et al., Indocyanine green fluorescence in second near-infrared (NIR-II) window, PLOS ONE, 2017
- Carr J.A., et al., Shortwave infrared fluorescence imaging with the clinically approved near-infrared dye indocyanine green, PNAS, 115, 2018
- Zhu S., Near-Infrared-II (NIR-II) Bioimaging via Off-Peak NIR-I Fluorescence Emission, Theranostics, 8, 2018
- Dou, et al., Design of Acticatable NIR-II Molecular Probe for In Vivo Elucidation of Disease-Related Viscosity Variations, Analytical Chemistry, 92, 2020
- Ge X., et al., Single Wavelength Laser Excitation Ratiometric NIR-II Fluorescent Probe for Molecule Imaging in Vivo, Analytical Chemistry, 92, 2020
- Liu S., et al., Constitutional Isomerization Enables Bright NIR‐II AIEgen for Brain‐Inflammation Imaging, Advanced Functional Materials, 30, 2020
- Xu P., et al., Molecular engineering of a high quantum yield NIR-II molecular fluorophore with aggregation-induced emission (AIE) characteristics for in vivo imaging, Nanoscale, 8, 2020
- Li D., et al., Monitoring the Real‐Time Circulatory System‐Related Physiological and Pathological Processes In Vivo Using a Multifunctional NIR‐II Probe, Advanced Functional Materials, 30, 2020
- Godard A., et al., Water-Soluble Aza-BODIPYs: Biocompatible Organic Dyes for High Contrast In Vivo NIR-II Imaging, Bioconjugate Chemistry, 31, 2020
- Li Yang, et al., Novel NIR-II organic fluorophores for bioimaging beyond 1550 nm, Chemical Science, 11, 2020
- Cai Z., et al., NIR-II fluorescence microscopic imaging of cortical vasculature in non-human primates, Theranostics, 10, 2020
- Hu Z., et al., First-in-human liver-tumour surgery guided by multispectral fluorescence imaging in the visible and near-infrared-I/II windows, Nature Biomedical Engineering, 4, 2020
- Wu D., et al., Extrahepatic cholangiography in near-infrared II window with the clinically approved fluorescence agent indocyanine green: a promising imaging technology for intraoperative diagnosis, Theranostics, 10, 2020