Calcium Imaging and Electrophysiology
Prof. Dieter Bruns, Dr. Yvonne Schwarz
Molecular Neurophysiology, Center for Integrative Physiology and Molecuar Medicine (CIPMM), University of Saarland, Germany
Background
The CIPMM Molecular Neurophysiology Lab studies the relationships between astrocytes and neurons, and their communication via the release of vesicles of neurotransmitters, including calcium (Ca2+). Prof. Dieter Bruns heads the lab, and researcher Dr. Yvonne Schwarz spoke about the research: "There is not much known in how astrocytes release neurotransmitters... they are prime candidates for governing neuronal function and even diminishing epileptic seizures. We are interested in how they release these transmitters in vesicles, and how the neurons will react." Calcium homeostasis is important for synaptic plasticity and learning, making this an active area of research.
Dr. Schwarz works with a combination of different samples, studying calcium signaling cascades with basic cell line models, then extrapolating into primary hippocampal co-cultures of astrocytes and neurons. These samples are stimulated, recorded, and imaged, combining calcium imaging and electrophysiology.
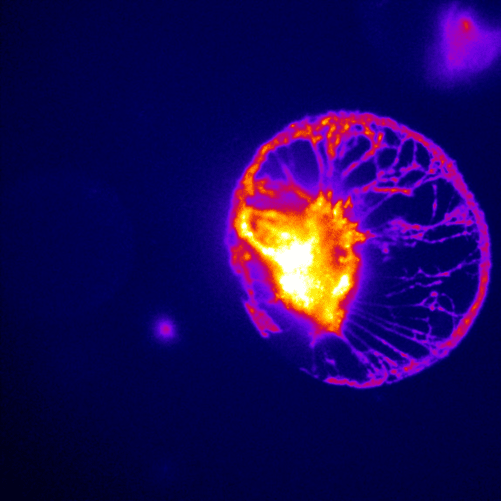
Figure 1: An image taken with the Prime 95B of glutamatergic neurons
stained with the glutamate sensitive fluorescent reporter iGluSnFR.
Challenge
The Molecular Neurophysiology Lab works with different neuroscientific applications, from optogenetics to calcium imaging, meaning that flexible imaging solutions are needed, such as high acquisition speeds needed when calcium imaging, in order to capture dynamic activity within the cells.
As Dr. Schwarz mentions: "We are trying to image with high speeds, the most challenging part is really combining electrophysiology with imaging, with electrophysiology coming from patch-clamping the neuron and calcium imaging coming from the GCaMP-transfected astrocytes, so it can be tough to combine the two and get them synced."
Dr. Schwarz has also been using an EMCCD camera for other experiments but was interested in sCMOS technology for a new imaging system.
[The Prime 95B] has a larger sensor and has a much better signal to noise than the EMCCD, that helps us a lot as I can image more cell populations within my astrocyte network, which is nice.
Dr. Yvonne Schwarz,Dieter Bruns
Solution
The high speed, high sensitivity, and large field of view of the Prime 95B presents a highly-flexible solution to the range of experiments occurring in the Molecular Neurophysiology Lab. As Dr. Schwarz said: "I have integrated [the Prime 95B] for the first time with an LED, and now I am running experiments and they work nicely, and I am actually pretty happy with it, I have to say... I haven't done any year-long experiments yet but it is a very good camera."
In terms of the Prime 95B field of view compared to EMCCD: "It is larger and it has a much better signal to noise than the EMCCD, and that helps us a lot actually, as I can image more different cell populations within my astrocyte network, which is nice." The low read noise, clean bias, and 95% QE of the Prime 95B results in a highly sensitive camera that improves on EMCCD technologies.
This application needs a combination of speed and sensitivity depending on the specific experiment, meaning the flexibility of the Prime 95B is very useful, using the high speed for calcium imaging in neurons, the high resolution for transmitter release in astrocytes, and the large field of view for general cell population experiments.
Learn More About The Prime 95B