Structured Illumination Microscopy (SIM)
Dr. Guy Hagen, Research Associate,
University of Colorado, Colorado Springs
Background
Dr. Guy Hagen, Research Associate from the University of Colorado, Colorado Springs creates high performance image reconstruction methods and open-source software to process super-resolution microscopy data. In 2014 Dr. Hagen released ThunderSTORM, an ImageJ plug-in for automated processing of photo-activated localization microscopy (PALM) and stochastic optical reconstruction microscopy (STORM) data.1 In 2016, he introduced SIMToolbox, a MATLAB toolbox for processing SIM data. It has the flexibility to process 2D and 3D images, including both optical sectioning and super-resolution applications, and can be used on data acquired from commercial systems.2 SIMToolbox also includes maximum a posteriori probability estimation (MAP-SIM), a super-resolution restoration method that suppresses out of focus light, improves spatial resolution, and reduces reconstruction artifacts.3 Dr. Hagen is currently developing live cell imaging using SIM, and is continuing to develop data analysis methods for super-resolution microscopy.
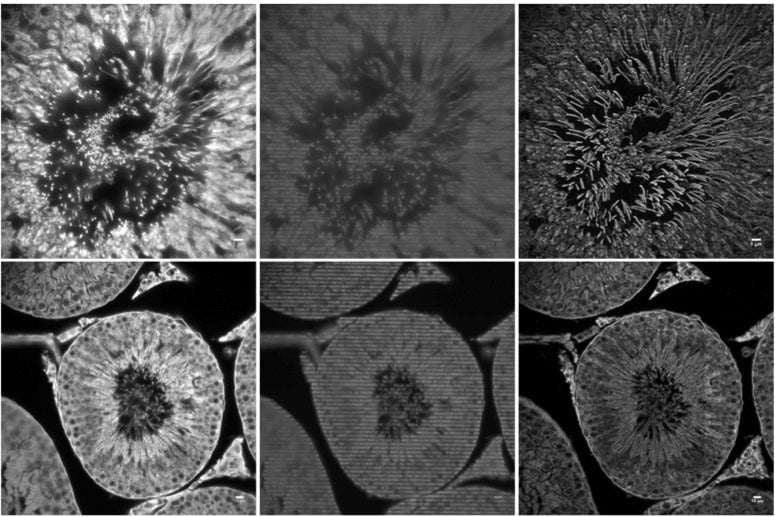
Cross section of a rabbit seminiferous tubule acquired using Dr. Hagen's structured illumination microscopy system and a
Prime 95B camera with a 100X/1.47NA objective (top row) or 40x/1.3NA objective (bottom row). Images were reconstructed
using SIMToolbox [2]. Conventional widefield fluorescence images (left) display out of focus light, raw SIM images (center)
display the illumination pattern, and the 3D SIM reconstruction (maximum intensity projection, right) shows high quality,
optically sectioned images.
Challenge
SIM is a widefield fluorescence technique that uses illumination patterns with high spatial frequency to illuminate samples. Algorithms applied to a combination of images taken with different phases and orientation of the illumination pattern are used to reconstruct a high-resolution image. Reconstruction of images routinely requires many images per focal plane. Fluorescent signals need to withstand photobleaching and the acquisition rate must be fast enough to observe live cell dynamics.
Imaging twice as fast with the Prime 95B [Scientific CMOS camera] is a great advantage. The reduction of pixel-to-pixel variance and the reduction of visible column structure in the camera greatly improves our results.
Dr. Guy Hagen
Solution
Because multiple images are required for the reconstruction of SIM data, a sensitive camera will allow for shorter exposure times and faster acquisitions, reducing phototoxic and photobleaching effects on samples.
The back-illuminated Prime 95B Scientific CMOS camera has a near perfect 95% quantum efficiency and large 11 µm pixels, making it extremely sensitive. The Prime 95B allowed Dr. Hagen to reduce exposure times to acquire a set of SIM images in about half the time required with typical sCMOS cameras.
Pixel-to-pixel and column-to-column gain variations which are present in typical sCMOS cameras can degrade SIM images during the reconstruction process. The Prime 95B reduces image artifacts by implementing several pixel noise filters to detect and correct dynamic fluctuations, and the static variation in gain and offset is calibrated for every pixel. Because of this, raw SIM images have a reduction in noisy pixels and visible readout lines, resulting in higher quality processed SIM images.
Learn More About The Prime 95B
References
- Ovesný, M., Krížek, P., Borkovec, J., Švindrych, Z., and Hagen, G. M. ThunderSTORM: a comprehensive ImageJ plugin for PALM and STORM data analysis and super-resolution imaging. Bioinformatics (2014), doi: 10.1093/bioinformatics/btu202
- Krížek P, LukeŠ T, Ovesný M, Fliegel K, Hagen GM. (2016). SIMToolbox: a MATLAB toolbox for structured illumination fluorescence microscopy. Bioinformatics (2016), doi: 10.1093/bioinformatics/btv576
- LukeŠ T, Krížek P, Švindrych Z, Benda J, Ovesný M, Fliegel K, Klíma M, Hagen GM. Three-dimensional super resolution structured illumination microscopy with maximum a posteriori probability image estimation. Opt Express (2014), doi: 10.1364/OE.22.029805