Sub-Cellular Neuronal Calcium Imaging
Prof. Barbara A. Niemeyer, Mr. Lukas Jarzembowski
DEPARTMENT CENTRE FOR INTEGRATIVE PHYSIOLOGY & MOLECULAR MEDICINE (CIPMM), SAARLAND UNIVERSITY, HOMBURG, GERMANY
Background
The lab of Prof. Barbara A. Niemeyer at CIPMM is interested in the molecular mechanisms underlying the regulation of calcium (Ca2+) signals in physiology and disease. The lab uses Zeiss microscopy systems, where Zen software is critical for the multi-user environment.
We spoke with a PhD student from the lab, Lukas Jarzembowski, “My research focuses mostly on the mechanisms of how calcium enters the cell, specifically how this is dysregulated in neurons and in the pre synapse for neural transmission. I am studying this at a single synapse level with primary hippocampal neurons using imaging and optical physiology tools.”
“One calcium entry route is through ion channels which interact with the endoplasmic reticulum (ER), I am using GCaMP fluorescent calcium sensors targeted to different cellular compartments to understand the role of this pathway in neural transmission.”

Figure 1: A video of primary hippocampal neurons expressing a pre-synaptic jGCaMP8f calcium indicator. Images show a whole neuronal network and the calcium activity after stimulation, where each dot is a single synapse. Video acquired with Kinetix22 in Sensitivity mode at 88 fps, 10 ms exposure time.
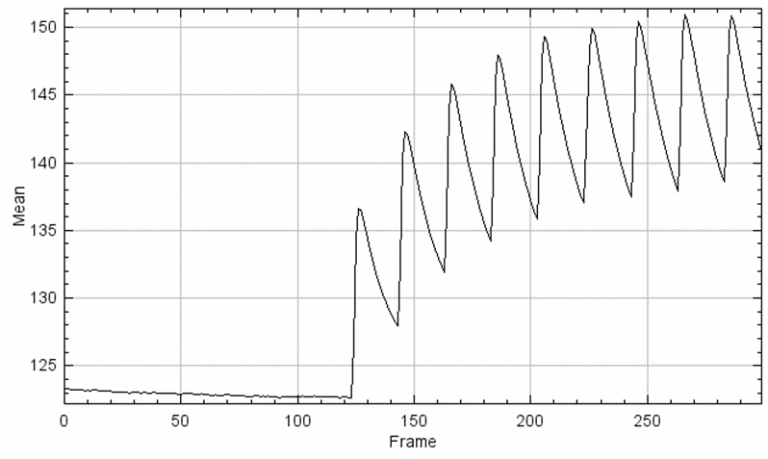
Figure 2: A Z-plot of activity over time, based on the samples in Figure 1. The graph shows a quiescent period in the beginning, followed by spikes of activity across the whole neuronal network, increasing in intensity.
Challenge
Mr Jarzembowski further described the imaging challenges of this application, “We are using a low light dose with our live primary neural cells, so the signal from GCaMP is quite low. Also, the pre-synapse is already quite tiny, and in the ER the pre-synapse is even tinier, so it is a very small volume containing very little GCaMP. Responses from individual synapses are also unpredictable, so I need to be able to record very faint signals from as many synapses as possible.”
“Ideally, I want to image at around 40 fps across the full FOV, but in the future, I may want to perform voltage imaging, which requires a much faster acquisition speed. As for resolution, I am looking for calcium signals in sub-cellular compartments, we have a Prime 95B on our Zeiss system which works perfectly fine, but the pixels are a little too large for our magnification and we want higher resolution, so we need a smaller pixel size.”
“We also have a Photometrics Evolve EMCCD and while it was sensitive, the FOV was way too small and it doesn’t make sense for me to do any experiments there.”
To this end, this application requires a camera that can maximize the field of view of the Zeiss imaging system, while also having high sensitivity in order to capture this very faint signal across the field at a sufficiently high speed, with sub-cellular resolution. Futureproofing this system for high-speed voltage imaging is also desirable, requiring a camera that can operate at 1000 fps or 1 kHz.
Solution
The Kinetix22 is an ideal solution for this application, featuring all the benefits of the Kinetix family of cameras while maximizing the FOV of the Zeiss microscope. Mr. Jarzembowski told us about his experience with the Kinetix22, “We tested the Kinetix22 on a wide range of different calcium sensors, such as GCaMP and glutamate signalling, all the way up to over 500 fps and it worked perfectly fine, all with a field of view that is very large, we can get the whole neuron on it which is what we need.”
“We are using Zen for our imaging; in our department we have lots of different users so the flexibility of Zen is critical. Hardware setup was simple with the Kinetix22 on our Zeiss system, we used a T-Mount adapter and this gives us the full frame of view, very useable and homogenous illumination with different light sources, no problem at all. Overall, the [Kinetix22] is working very well.”
